Modern Countercurrent Chromatography
LCGC Europe
CCC is an excellent alternative to avoid the problems associated with solid-phase adsorbents, which include irreversible adsorption of sample and the need to replace expensive columns. As a result, CCC is gaining popularity as a purification tool.
Countercurrent chromatography (CCC) is basically the liquid–liquid partition of a sample between two immiscible liquid phases. This is the same separation principle as separating two mixtures using a simple separating funnel experiment. The technique developed from countercurrent distribution, which was investigated in the 1940s to provide separations without a solid chromatographic support. The term "countercurrent chromatography", although generally accepted nowadays, is in fact a misnomer because most systems involve the passage of one phase (the mobile phase) of a biphasic solvent system through a second non-mobile (stationary phase) component of the solvent system.

This article will provide a review of CCC because the technique is at last becoming more widely accepted by the scientific community. Reliable instruments are now being produced and applications in university and industrial circles are increasing.1
CCC Techniques
There are two basic CCC techniques, each with its own instruments: (a) CCC with coiled tubes and (b) CCC with cartridges or discs.
CCC with coiled tubes: CCC with coiled tubes involves the rapid rotation of a polytetrafluoroethylene (PTFE) or Tefzel tube wrapped around a drum/bobbin (Figure 1) and is known as centrifugal countercurrent chromatography (CCCC) or high-speed countercurrent chromatography (HSCCC). This mainly involves apparatus with a variable gravity field produced by a double-axis gyratory motion and seal-free introduction of solvent onto the column. One of the most important experimental parameters (β) is defined as the ratio of the coil radius (r) to the orbital radius of revolution (R). Almost 100% of the column space is used for the mixing of the two immiscible phases in this hydrodynamic system.
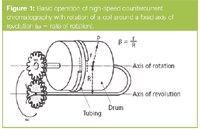
Figure 1
As far as instruments are concerned, Dr Y. Ito from the National Institutes of Health (NIH) in Bethesda, Maryland, USA has invented most of the prototypes2 but a variety of instruments are now commercially available.
CCC with cartridges or discs: The CCC technique involving cartridges or discs produces a constant gravity field by single-axis rotation and solvent is supplied through rotating seals. Separation occurs in the cartridges or discs. A hydrostatic equilibrium system results, which can be compared to a static coil. If the disc is filled with the stationary phase of a biphasic solvent system and then the other phase is pumped through the disc at a suitable speed, a point is reached where no further displacement of the stationary phase occurs and the apparatus contains approximately 50% of each of the two phases. Steady pumping-in of the mobile phase results in the elution of mobile phase alone. This basic system uses only 50% of the efficient column space for the actual mixing of the two phases. A range of rotating disc instruments are available commercially.
Applications
CCC is an excellent alternative to avoid the problems associated with solid-phase adsorbents, which include irreversible adsorption of the sample and the need to replace expensive columns. As a result, CCC is gaining popularity as a purification tool for natural products, especially in the bioassay-guided fractionation of plant-derived compounds.
For example, for the preliminary fractionation of large quantities of crude plant material, a methanol extract (26 g) of Euclea mayottensis (Ebenaceae) root bark was introduced onto a Pharma-Tech CCC-1000 (Pharma-Tech, Baltimore, Maryland, USA) with a column capacity of 650 mL. This was separated into several fractions, three of which contained the major naphthol glycosides responsible for the antifungal activity of the extract (Figure 2). A final gel filtration step on Sephadex LH-20 (Pharmacia, Uppsala, Sweden) with methanol–water mixtures was sufficient to obtain the pure compounds. An advantage of CCC is the ability to reverse the flow direction and interchange the mobile and stationary phases ("reversed-phase" operation). In the case of E. mayottensis, changing the mobile phase to the upper layer of the solvent system eluted the most polar components of the extract.
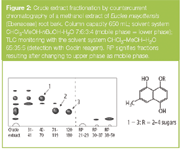
Figure 2
At the other end of the scale, CCC is also very useful for the separation of small amounts of sample and/or the final purification of fractions. For this purpose, short coils on small bobbins rotating at high speed are used. These have a capacity ranging from about 10–50 mL (with tubing of internal diameters of 1 mm or less). Loading factors and the timescale of the separations make this an attractive alternative to semi-preparative high performance liquid chromatography (HPLC).
Although the efficiency — which is represented by the number of theoretical plates — cannot match that of HPLC, the high selectivity and the high stationary phase to mobile phase ratio more than compensates. In HPLC, around 20% of the volume of the column is a stationary (bonded) phase around the silica support, available for interaction with a solute, while in CCC the figure for the stationary phase content can be as much as 80%.
To illustrate the application of CCC in a small-scale operation, the isolation of a flavonoid glycoside with inhibitory activity against the enzyme acetylcholinesterase (AChE) will be described. Inhibitors of AChE provide potential therapeuticals for the management of Alzheimer's disease and efforts are presently being made to find more effective drugs with this particular mode of action.3
A screening programme found that the methanol extract of the leaves of the common ornamental plant Buddleja davidii (Buddlejaceae) possessed inhibitory activity against AChE. The active compound was isolated in one step by centrifugal countercurrent chromatography on a Dynamic Extractions (Slough, UK) mini centrifuge instrument with a 17 mL bobbin (Figure 3). This was identified as the flavonoid linarin, with a detection limit (DL) of 10 ng in a bioautographic thin layer chromatography (TLC) assay for AChE inhibition, which is at the same level as the known active compound galanthamine. Injection of 20 mg crude extract gave 2 mg of pure flavonoid glycoside and the total elution time of 60 min is comparable with the timescale for semi-preparative HPLC. There are many other CCC applications and these can be found in the following review articles1,4 and monographs.2,5,6
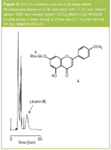
Figure 3
CCC Techniques
CCC is very flexible and can be used in normal phase, reversed-phase and gradient elution modes. Elution extrusion, pH zone refining and dual-mode elution also add to the versatility of the technique.
pH zone-refining: pH zone-refining can be used for ionizable compounds. This method relies on the principle that charged molecules prefer aqueous phases and uncharged molecules prefer organic phases. By the judicious use of basic organic phases and acidic aqueous phases (or vice versa), the compounds in the stationary phase are eluted by the mobile phase according to their pKa values and solubilities. This method allows a large increase in sample loading capacity.
Elution extrusion: Elution extrusion takes advantage of the fact that the sample may be fully separated inside the column before being eluted. When the separation run reaches a certain point, the mobile phase is stopped and stationary phase is pumped-in to extrude the column contents. The advantage of this method is that solvent consumption is significantly reduced.
Dual-mode elution: The aqueous phase is initially used as the mobile phase and after a certain lapse of time, the organic phase is pumped as the mobile phase. This switching procedure is repeated until the desired resolution for separation is achieved. The advantage of this method is that compounds with a strong affinity for the original stationary phase can be separated rapidly, rather than waiting a long time for them to elute in the mobile phase.

Keynotes
Machines with a capacity of up to 50 L of solvent are now on the market, implying that sample quantities in the 100 g range can now be handled in a single injection. This is an exciting development because it opens the way to produce the large quantities required by industry. For example, separation of the two stilbenes honokiol and magnolol from Magnolia officinalis (Magnoliaceae) was possible at a productivity rate of 430 mg/min of 99.9% pure compounds.7
Conclusions
Countercurrent chromatography (CCC) is an all-liquid separation technique which is particularly useful for the isolation of natural products. It can be successfully used to prepare laboratory-scale to industrial-scale quantities. For this reason, CCC is of interest to research groups investigating bioactive natural products and to pharmaceutical and chemical industries. The technique is versatile and can be operated in a variety of modes. This flexibility of operation and the absence of a solid-phase support give CCC certain advantages over adsorption methods. The most important item is the total recovery of the sample introduced into the CCC instrument.
Andrew Marston is senior research scientist in the Laboratory of Pharmacognosy and Phytochemistry, School of Pharmaceutical Sciences, University of Geneva, Switzerland. His interests include the preparative isolation of bioactive natural products and the investigation of saponins.
Kurt Hostettmann is director of the Laboratory of Pharmacognosy and Phytochemistry, School of Pharmaceutical Sciences, University of Geneva, Switzerland. His interests revolve around the isolation of lead compounds from natural sources and include both analytical and preparative chromatographic methods.
References
1. A. Marston and K. Hostettmann, J. Chromatogr. A, 1112, 181–194 (2006).
2. W.D. Conway, Countercurrent Chromatography: Apparatus, Theory and Application (VCH Publishers, New York, USA, 1990).
3. K. Hostettmann et al., Current Organic Chemistry, 10, 825–847 (2006).
4. Y. Ito, J. Chromatogr. A, 1065, 145–168 (2005).
5. K. Hostettmann, A. Marston and M. Hostettmann, Preparative Chromatography Techniques: Application in Natural Product Isolation (Springer-Verlag, Berlin, Germany, 1998).
6. A.P. Foucault, Centrifugal Partition Chromatography (Marcel Dekker Inc., New York, USA, 1995).
7. L. Chen et al., J. Chromatogr. A, 1142, 115–122 (2007).

Evaluating Body Odor Sampling Phases Prior to Analysis
April 23rd 2025Researchers leveraged the advantages of thermodesorption, followed by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS), to compare and assess a variety of sampling phases for body odor.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











