SFC–GCxGC to Analyse Matrices from Petroleum and Coal
LCGC Europe
SFC–GCxGC is shown to be useful in fossil fuel analysis. Practical examples for the analysis of complex matrices are described.
Badaoui Omais,1 Thomas Dutriez,1 Marion Courtiade,1 Nadège Charon, Jérémie Ponthus,1 Hugues Dulot1 and Didier Thiebaut,2
1IFP Energies Nouvelles, Solaize, France,2ESPCI, PECSA UMR CNRS 7195, Paris, France.
Supercritical fluid chromatography (SFC) is a valuable tool prior to comprehensive two-dimensional gas chromatography (GC×GC) analysis to investigate the composition of petroleum vacuum gas oils (VGOs) and selectively analyse phenolic compounds in coal-derived middle distillates. In the first case, a multidimensional SFC system is used to separate saturates, aromatics and polar compounds in a VGO sample, and in the second case a single ethylpyridine stationary phase is sufficient to selectively separate phenols.
Traditional fossil fuel sources are dwindling and new ways to isolate fuels from existing sources are vital. Two methods look particularly promising: converting heavy petroleum fractions into lighter fractions and transforming coal-derived liquids into synthetic fuels that fit engine requirements. These two matrices involve two separate problems. Converting heavy fractions, such as vacuum gas oils (VGOs), into specific groups is difficult because compounds contained in heavy petroleum fractions must be transformed into lighter compounds, and coal-derived middle distillates have a high phenol content which must be precisely determined if these distillates are to be processed further (1).

These problems can be solved by using a high-resolution technique, such as multidimensional chromatography, which was developed to overcome the limited peak capacity of one-dimensional chromatographic methods (2).
Although comprehensive two-dimensional gas chromatography (GC×GC) (3) is popular in many sectors, including food, biology and environmental, it is still difficult to cope with some complex samples in terms of group-type selectivity and peak capacity. This difficulty has been highlighted in recent work on analysing diesel fuels (4) and heavy petroleum fractions, such as VGOs (5,6). The lack of group-type selectivity is often a result of the limited choice of polar stationary phases. To overcome this problem multidimensional systems incorporating a separation using a dense mobile phase have been suggested as a solution, including a SFC×GC system (7) and an LC×GC system (8–10).
When considering on-line hyphenation of an LC system with a GC or GC×GC system, the key technical issue is the evaporation of the LC mobile phase which must induce a limited reinjection band for GC.
The stop-flow mode via adapted valve-based- or syringe-based interfaces is preferred because of its flexibility (11,12). Although remarkable results have been obtained for edible oils (12) and petroleum samples (11), the total running time remains a major obstacle.
SFC using CO2 as the mobile phase seems more compatible with a gas phase dimension than an LC dimension. The improved compatibility of SFC can be attributed to the expansion stage from the supercritical state to the gas state without any loss of the products of interest. Additionally, SFC separations can provide higher selectivity because of the variety of solvent and solutes, and stationary phase interactions, and because they are often faster than LC separations (13).
The hyphenation of SFC with GC has been already demonstrated by the decompression of the supercritical fluid via a restrictor inserted into the GC injection port (14). This was applied using SFC–GC (15). The symbol "–" defines a heart-cutting hyphenation between SFC and GC, and The "×" symbol in SFC×GC (16,17) defines a comprehensive coupling.
Capillary SFC×GC was demonstrated by the pioneering work of Liu et al. (16). The effluent from the first SFC column was received continuously by the second GC column through a thermal desorption modulator (16). Venter et al. proposed a stop-flow SFC×GC system for oil samples analysis (7) with a fast GC dimension.
An additional dimension involving SFC appears to be very complementary to GC×GC systems.
A SFC–GC×GC (18) configuration was designed for the analysis of a diesel sample. The sample was separated into two fractions, which were directly transferred into two distinct GC×GC column sets (SFC–twin-GC×GC). However, the SFC fractions were not managed by a special interface. Therefore, an interface for multidimensional chromatography between SFC and GC was developed. The design provides an integrated solution for the hyphenation of a SFC system and GC×GC system, and can be operated as an SFC system, an SFC–GC system, an SFC–GC×GC system and also as an SFC×GC×GC system (19).
This paper aims to show how this system was used to unravel the complexity of heavy VGOs and to separate phenolic compounds in coal-derived middle distillates.
Material and Methods
Samples: Vacuum Gas Oils
Test Mixture 1 (TM1): The separation of vacuum gas oils by SFC was first studied with a synthetic test mixture prepared in carbon disulphide (Table 1).
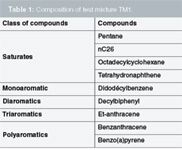
Table 1: Composition of test mixture TM1.
Sample: A vacuum gas oil of an Arian light feedstock was supplied by IFP Energies Nouvelles (Lyon, France) (Table 2). Before injection, it was previously diluted in CS2 with a 1:3 w/w ratio.
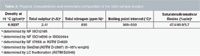
Table 2: Physical characteristics and elementary composition of the VGO sample studied.
Direct coal liquefaction middle distillate
Test mixture 2 (TM2): To evaluate the potential of different columns to separate phenolic species, a model mixture representative of a coal- derived middle distillate was prepared. Six families of chemical were selected because they were identified in previous works (1,20): Paraffinic, naphthenic, aromatic, furanic, phenolic and nitrogen-containing compounds. For each chemical family a light and a heavy compound was selected (Table 3). The solvent used for the test samples was ethyl acetate and was supplied by VWR (Fontenay-sous-bois, France). Chemicals were provided by different suppliers: Sigma-Aldrich (Saint Quentin Fallavier, France), Merck (distributed by VWR), Alfa Aesar (Schiltigheim, France), TCI (Zwijndrecht, Belgium) and Fluka (Saint Quentin Fallavier, France).
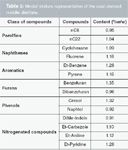
Table 3: Model mixture representative of the coal-derived middle distillate.
Sample: The investigated coal-derived oil was provided by IFP Energies Nouvelles. It consisted of a middle distillate (boiling points range = 200–350 °C) of a coal oil produced by direct liquefaction. The sample used in this study had a hydrogen content of 11.4% w/w [by nuclear magnetic resonance (NMR) spectroscopy], a nitrogen content of 0.24% w/w (ASTM D4629), a sulphur content of 0.0072% w/w [provided by X-Ray Fluorescence (XRF)], and an oxygen content equal to 0.80% w/w. The refractive index (RI) and density of the sample were 1.5129 (ASTM D1747) and 0.9330 g/cm3 (NF EN ISO 12185) respectively.
Systems
The SFC system: Samples were injected in the instrument through a switching valve equipped with an injection loop of 5 µL. The mobile phase, carbon dioxide (Alphagaz CO2 SFC grade, Alphagaz, Mitry-Mory, France) was delivered by a PU-2080-CO2 pump (Jasco, Bouguenais, France) at a constant flow-rate of 2.5 mL/min. The backpressure was regulated by a BP-2080 pressure regulator (Jasco). The SFC column(s) and tubular connections were introduced in an in-house modified gas chromatograph (GC6890, Agilent Technologies, Massy, France). All two-positions switching valves were managed via a pneumatic controller (6 ports AC6W, VICI, Schenkon, Switzerland). All tubular connections were in stainless steel (1/16" × 0.13 mm, 0.005') (Interchrom, Montluçon, France). All of the valve heads were placed in regulated heat chambers located at the top of the GC chromatograph. The SFC separation was monitored with a flame ionization detector (FID) set at 350 °C. For this purpose, the total supercritical flow-rate was split (about 1:100) by an integral restrictor made in-house (21) using a stainless steel cross union ZT1C (1/16'', 0.25mm, VICI). The SFC was controlled by independent Chemstation software (Agilent Technologies).
The GC×GC system: The GC×GC system consisted of an in-house modified 6890 chromatograph (Agilent Technologies) equipped with dual-stage carbon dioxide jets (22). To perform modulation simultaneously for two column sets (SFC–twin-GC×GC), the columns holder supplied by Thermo (Milan, Italy) was adapted on top of the chromatograph. The 2D modulation was performed at the beginning of the second columns and the modulation period was set at 30 s.
The 2D modulation was managed by a controller built in-house. The GC×GC instrument was equipped with two split/splitless injectors and two FIDs (370 °C, 100 Hz). H2 and air flow-rates were 35 and 400 mL/min respectively. Two GC×GC columns sets were installed. Helium (99.99%) was used as the carrier gas at constant adapted pressure (23). During the transfer of SFC fractions into the GC×GC system, the trapping temperature was set at –30 °C.
Once the SFC fractions were trapped on the GC phases, the oven returned to the initial temperature (100 °C for VGOs and 50 °C for coal-derived products) at 20 °C/min and the analytical ramp was controlled at 2 °C/min up to 370 °C for VGOs analysis and to 280 °C for coal- derived products analysis.
The data processing of 2D contour plots was performed by 2DChrom (Thermo) after raw data transfer as CSV-files. The control of the (i) GC×GC dimension, (ii) the interface including the valves management and (iii) the data acquisition was performed using independent Chemstation software (Agilent Technologies).
The Interface: An interface allowing the intermediate sampling of SFC fractions into loops was designed and implemented. The designed interface is shown in Figure 1. It should be noted that none, one, multiple or all of the supercritical effluents coming from the SFC part can be distributed into the GC×GC system.
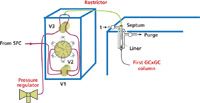
Figure 1: Schematic of the designed interface for SFCâGCÃGCÃGC.
The designed interface was introduced in a highcapacity oven supplied by Interchrom (Montluçon, France). To avoid any change in the physical state of the supercritical effluents, the same temperature and pressure conditions as the SFC separation ones were applied across the whole interface. Therefore, all of the tubular connections and the valve heads were placed in the oven using long metal actuators between the valve head in the oven and the motor out of the oven (VICI).
This interface was based on a set of three main valves: V1, V2 and V3. The first two valves (6 ports AC6W, VICI) were managed using a pneumatic controller. V2 is a 12-port valve EMT2CST6UW (1/16", 0.4 mm, 0.016'), supplied by VICI and is resistant to high pressure (up to 345 bar) and temperature conditions (up to 75 °C); six HPLC sampling loops (model SLKCW, VICI) were adapted using the 12 ports of the multi-positions valve. The management of V2 was performed by a microelectric actuator (EMTCA-CE, VICI) directly piloted by the Chemstation software (Agilent Technologies).
Technological aspects: The function of the V1 valve was the sampling management of supercritical effluents coming from the SFC part towards valve V3 (closed) or the multi-positions valve V2.
V2 allowed the sampling or draining of supercritical effluents, by successively switching the rotor position, that is each loop, allowing for their transfer into or from a loop. The volume of the sampling loops depended on the SFC separation and the sampling of supercritical fractions. The optimal volume for the draining of loops was set at 1.5 times their physical volume and at a minimum of 10 mL.
The V3 valve allowed the transfer of supercritical fractions into the GC×GC system, by splitting the total flow-rate using a derivation combined with a restrictor directly inserted in the GC×GC injector (14). V3 was closed when just implementing a single SFC separation, as well as performing a GC×GC run, that is fractions already transferred, to prevent leaks from the inlet septum. Supercritical fractions were expanded at the restrictor tip inside the GC injector, and then solutes were cryo-focused on the first centimetres of the first GC×GC column.
After the transfer of each SFC fraction, the purge valve of the injector was opened to drain the residual CO2 out of the system. When two different columns sets were available in the same GC×GC oven for SFC–twin-GC×GC operation (two different injectors and detectors were used for GC×GC), for each fraction to be transferred from the SFC system, the selection of the GC×GC columns set to be connected to the SFC effluent was performed using another valve (V4) placed between V3 and the two restrictors.
Concerning the transfer of supercritical effluents, the interface described by Levy et al. (14) has been implemented, that is the decompression of the supercritical fluid via a restrictor inserted into the GC injection port. Conditions were maintained at –30 °C for the trapping temperature, 350 °C for the GC injector temperature and 13 mL/min for the restrictor flow-rate. Supercritical mobile phase was depressurized using the integral restrictor before the trapping of solutes on the first centimetres of the cold first column. The splitless mode was used during transfer. Once the transfer was done, the purge valve was opened to release residual CO2 and the oven returned to the initial temperature (100 °C for heavy oils, 50 °C for coal-derived liquids) at a ramp of 20 °C/min; then the analytical ramp could start.
Stationary Phases
Investigated SFC Columns: The selectivity of various stationary phases (Table 4) was studied with the test mixtures MT1 and MT2. Experiments were performed at 35 °C and the backpressure was regulated at 250 bar.

Table 4: Stationary phases studied for the SFC separation.
GC Column Combinations
VGOs: For the application for VGOs, a first 2D-GC column set was dedicated to SFC fractions composed of saturated compounds. It consisted of a combination of a non-polar first column: DB1-HT (dimethylpolysiloxane, J&W Scientific, Folsom, USA) (10 m × 0.32 mm, 0.1 µm) and a mid-polar second column, BPX-50, (50% phenyl)polysilphenylene-siloxane, (SGE, Courtaboeuf, France), (0.8 m × 0.1 mm × 0.1 µm). The second column set was specifically dedicated to SFC fractions composed of unsaturated and polar compounds. It consisted in a combination of a DB1-HT (10 m × 0.32 mm, 0.1 µm) and a BPX-50 (1.45 m × 0.1 mm × 0.1 µm).
Coal-derived Middle Distillates: For the analysis of phenolic compounds the column set consisted in a polar Solgelwax (30 m × 0.25 mm × 0.25 µm) in the first dimension coupled with a DB-1 (1 m × 0.1 mm × 0.1 µm) in the second dimension. This configuration involving a polar column in the first dimension and an apolar one in the second dimension was supposedly non-orthogonal because retention mechanisms of each dimension are not independant. However, this configuration enabled the separation of phenols from hydrocarbons, but these species could not be classified by carbon atom in the chromatogram in the same way as orthogonal configurations.
Results and Discussion
Selectivity of SFC Stationary Phases
VGO: The implementation of a SFC separation before the GC×GC separation must improve on the lack of group-type selectivity observed for the high temperature 2D-GC (HT-2D-GC) analysis of VGOs (24). The resolution between saturated and unsaturated compounds and the selectivity by aromatic rings number needs to be improved in particular. Consequently, a stationary phase study was performed by one- and multidimensional SFC.
1D-SFC: A "conventional" stationary phase [used in adsorption or normal phase (NP) chromatography] is selective enough to perform a group-type SFC separation of light matrices, such as gasoline (25) and even diesel oils (13,26) in saturates, monoaromatics or diaromatics. However, owing to a higher complexity, the chromatographic resolution can be reduced for some diesels (27). To check the possible applications of 1D-SFC to heavy fractions, such as VGOs, several promising selective stationary phases were studied. This was based on potential high π–π or dipolar interactions (28,29) by the stationary phases. After SFC–FID experiments on the test mixture TM1, chromatographic resolutions were calculated between different couples of compounds (Figure 2).
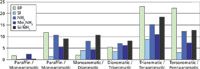
Figure 2: Resolutions between model compounds of TM1 as a function of stationary phases by SFCâFID.
Polyethyleneglycol (PEG) and polystyrene/divinylbenzene (PS-DVB) stationary phases produced too high retention times or compatibility problems with SFC conditions to be viable. Ethylpyridine, silica, aminopropyl-silica and dimethylaminopropyl-silica stationary phases produced low resolutions between nC26 paraffin and octadecylcyclohexane. High resolutions were obtained with ethylpyridine for saturated/aromatic compounds and also for highly aromatic structures. However, silica and aminopropyl-silica phases connected in series produced the best results for test mixture TM1 (Figure 3).
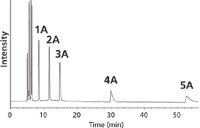
Figure 3: SFCâFID chromatogram of TM1 with Si/NH2 (150 bars, 35 °C). The number of rings in indicated for aromatic compounds.
Multidimensional SFC: A silver-modified silica (Ag/Si) was considered because conventional stationary phases did not provide the expected resolutions. This phase enables charge transfer chromatography (30) by very high π–π interactions with the empty orbital of the silver atom. Frequently, it is used for triglycerides (31) or unsaturated compound analysis (including olefins) in gasoline or diesel (32). Actually, its high π–π interactions are strong enough to fully separate saturated and unsaturated compounds, including compounds in crude oils (33). Therefore, based on previous work (34), the original association between partition chromatography (cyanopropylsilica, CN) and silver-modified silica was implemented. Eventually, a NH2 stationary phase could be added, via valve 7, to improve the separation of aromatic compounds according to the number of aromatic rings. A schematic representation of their combinations is shown in Figure 4.
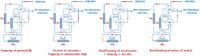
Figure 4: Schematic representation of (1) Association of the CN and Ag/Si columns and (2) Association of the CN, Ag/Si and NH2 columns. Conditions are detailed in the experimental section.
As a result of high dipolar interactions, the most polar compounds were retained on the CN column, whereas unsaturated and saturated compounds eluted towards the silver modified phase. Using valve V6, the CN column was then isolated according to retention times monitored by preliminary SFC–FID experiments using the CN column [Figure 5(a)]. The commutation time of V6 was determined by considering the elution of benzo(a)pyrene in the unsaturated fraction [10 mins in Figure 5(a)] and that of highly retained solutes (especially nitrogen-compounds) in the polar fraction. Regarding the Ag/Si column, saturated compounds were directly eluted towards the FID and the interface, whereas unsaturated analytes were retained.

Figure 5: SFCâFID chromatograms (250 bars, 65 °C) of (a) Model mixture with CN, (b) Model mixture with CN and Ag/Si, (c) The vacuum gas oil with CN and Ag/Si. See conditions in experimental section.
Once all the saturates were eluted, the unsaturated fractions were backflushed towards either the NH2 column for a further aromatic group separation or to the FID and the interface. Finally, backflushing of the CN phase released the polar fraction.
The performance of this column combination is illustrated with the separation of the test mixture [Figure 5(b)] and vacuum gas oil [Figure 5(c)]: a clear distinction between peaks of saturated, aromatic and polar hydrocarbon was obtained on both test and real samples.
Coal-derived materials: The objective of this part was to select from three stationary phases the most appropriate one to separate phenolic compounds from the rest of the matrix (consisting of hydrocarbons and nitro-containing compounds). Three phases were selected according to their interactions with phenolic compounds. It is known that hydroxyl groups can form H-bonds with the various lone pairs of the stationary phases (Figure 6).
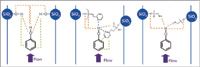
Figure 6: Interactions between phenols and stationary phases. (a) Silica, (b) Pyridine, (c) Cyanopropyle.
A silica phase is very retentive towards phenolic compounds because it implies a double H-bond as described in Figure 6(a). Additionally, with this phase, interactions with the aromatic group is important. The ethylpyridine stationary phase involves O-H... N hydrogen bonds, which are stronger than the O-H... O ones (29 kJ/mol against 21 kJ/mol); additionally, this phase is composed of three Si-O groups implying both lone pairs of electrons that will form additional H-bonds with the labile proton; π interactions between the two aromatic groups can also be formed. For all these reasons, the ethylpyridine stationary phase should be very retentive towards phenols and is a possible solution to separate them from the hydrocarbon matrix. The cyano stationary phase was also studied because it implies H-bonds with phenols as it possesses one lone pair on the nitrogen atom and two others on the oxygen one.
To model the separation of phenols with these three phases, cresol and naphthol were injected in the SFC–FID system. The elution times of these species are reported in Figure 7. Hydrocarbons and N-compounds were also injected to evaluate the selectivity towards hydrocarbon groups and phenols. As expected, hydrocarbons rapidly eluted from the column. N-compounds were strongly retained by all these phases especially for the silica, which can form H-bonds with indoles, aniline, pyridine and carbazoles.

Figure 7: Elution zones of different chemical classes representative of a coal-derived middle distillate.
Considering the elution zones of all the chemical families investigated, the ethylpyridine stationary phase was selected to separate phenols. A coal liquefaction middle distillate was then injected in the same experimental conditions and the chromatogram is presented in Figure 8. A broad peak was observed in the 40–46 min zone of the chromatogram. The model mixture injection (Table 3) shows that phenols elute in this specific zone.

Figure 8: SFCâFID chromatogram of a coal-derived middle distillate (Pyridine column).
Applications
VGOs: Heart-cutting experiments using the SFC–GC×GC system were performed on the VGOs (Figure 9). Saturated, unsaturated and polar fractions were transferred into the GC×GC system by the interface described in the experimental section.

Figure 9: 2D contour plots for the SFC-2GCÃGC analysis of the vacuum gas oil. (a) Saturates, (b) Unsaturates, (c) Polars.
Regarding the saturated fraction of the VGOs, the results are consistent with those obtained with off-line LC–(HT-2D-GC) analysis (35) with a clear distinction between polynaphthenic solutes and iso-paraffins. Aromatic groups can be clearly distinguished on the unsaturated 2D contour plot of the VGOs. Concerning the polar fraction, two distinct 2D elution zones were clearly observed, which most likely correspond to aromatic heteroatomic (N-, S-) compounds.
The assignment of 2D elution zones on the 2D contour plots was facilitated by the use of test mixtures, except for the polar fraction. It is worth pointing out that the families would coelute in GC×GC without prior SFC separation.
As the identification of the polar fraction was not confirmed yet, quantitative analysis was only performed on the saturated and unsaturated fractions (Table 5). The processing of the quantification step was performed by a 100% normalization of each 2D contour plot. A global correction was performed using the fractions ratio determined by SFC separation monitored using the FID. As SFC was implemented before GC×GC, more families could be determined compared with high temperature GC×GC (HT–GC×GC) and SAR-MS (saturates, aromatics and resins separation before MS analysis).
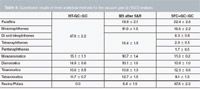
Table 5: Quantitative results of three analytical methods for the vacuum gas oil (VGO) analysis.
Coal-derived middle distillates: SFC was coupled to GC×GC to obtain a detailed characterization of coal-derived middle distillates described in the previous separation of phenolic compounds. Previous work in GC×GC showed that the column combination of polyethylene glycol × polydimethylsiloxane lead to a specific elution zone corresponding to phenolic compounds as shown in Figure 10(a) (36). When combined with the ethylpyridine SFC dimension, the GC×GC separation leads to a chromatogram where only this specific zone appears [Figure 10(b)]. This highlights the excellent fractionation of phenolic compounds obtained in the first SFC dimension.

Figure 10: 3D chromatograms of a coal-derived middle distillate by GCÃGCâFID (Solgelwax à DB-1). (a) Without a SFC pre-separation, (b) with a SFC pre-separation.
Conclusion
The advantages of hyphenating the SFC dimension before GC×GC analysis have been demonstrated to investigate the composition of complex matrices, such as VGOs, or to selectively separate phenolic compounds from oil-derived samples. In the first case a multidimensional SFC configuration enables a separation of saturates, aromatics and polar compounds. These fractions can be analysed further by GC×GC to access a detailed characterization. In the second case, only one SFC column is required to separate phenolic compounds from hydrocarbons in a coal-derived middle distillate and this fraction can be transferred directly to the GC×GC system.
The selectivity of the ethylpyridine stationary phase is so high that a one-dimensional GC system should be sufficient to access for a detailed characterization of phenols.
SFC has major benefits for separating oil-derived compounds. It can be performed for group-type separations with a FID and complement GC separations for heavy compounds analysis. SFC can easily be hyphenated to GC and GC×GC systems to obtain a highly detailed characterization of medium volatility oil samples.
Badaoui Omais is a PhD student at IFP Énergies Nouvelles, France. He currently works on the speciation of oxygen in complex matrices such as biomass pyrolysis oils and direct coal liquefaction products. His research focuses on the transformation of these products into biofuels and synthetic fuels.
Marion Courtiade graduated from the school of Chimie Physique and Electronique (CPE) in 2004. She obtained her PhD from Lyon University, which deals with trace analysis of pesticides in environment. Since 2007, Dr Courtiade has been head of the GC laboratory at IFP Energies Nouvelles and is an expert in characterization by comprehensive gas chromatography.
Jérémie Ponthus graduated in 2002 and obtained his PhD dealing with Metastable Atom Bombardment Mass Spectrometry for petroleum-related molecules characterization from the University of Paris (UPMC), France in 2005. He then joined IFP Energies Nouvelles where he is now heads the Mass Spectrometry laboratory. His research is focused on the FT/MS analysis of complex mixtures from heavy crude oils, biomass and coal.
Didier Thiébaut, PhD is senior scientist at CNRS. His research at ESPCI-ParisTech focuses on multidimensional chromatographic techniques (LC, GC and SFC) and their hyphenation to selective detection and sample fractionation techniques to improve comprehensive analysis of complex samples.
Thomas Dutriez has a PhD in analytical chemistry from the University of Paris and works as scientist at DSM Resolve, The Nederlands, since 2011. His research at IFP Energies Nouvelles, is focused on the characterization of vacuum gas oils by multidimensional chromatographic techniques using GC×GC, for supporting hydrotreatment modelling.
Nadège Charon graduated from the CPE Lyon School in 2002 and obtained her PhD dealing with kinetic modelling of hydrotreatment of petroleum vacuum gas oils from ENS Lyon, France (2005). She then joined the Physics and Analysis Division of IFP Energies nouvelles. Her current research interests are focused on the multi-techniques analytical characterization of products derived from biomass and coal resources.
Hugues Dulot is a senior research engineer at the Development Center of IFP Energies nouvelles. He holds an MS and a PhD in Chemical Engineering from the University of Nancy (France), where he studied the modelling of separation-reaction processes. He joined the IFPEN research centre in 2001, where he works on the kinetic and process modelling of hydrotreating and hydrocracking units. From 2009 onwards, he has lead the R&D catalysts and process development on hydrotreating and hydrocracking of vacuum distillates feeds.Concurrently, he continued his basic research through the supervision of several PhD. theses.
References
1. B. Omais et al., Energy Fuels, 24(11), 5807–5816 (2010).
2. J.C. Giddings, J. High Resolut. Chromatogr., 10(5), 319 (1987).
3. H.J. Cortes et al., J. Sep. Sci., 32(5–6), 883 (2009).
4. F. Adam et al., J. Chromatogr. Sci., 45, 643. (2007).
5. T. Dutriez et al., J. Chromatogr. A, 1216, 2905 (2009).
6. T. Dutriez et al., Fuel, 89(9), 2338 (2010).
7. A. Venter and E.R. Rohwer, Anal. Chem., 76(13), 3699 (2004).
8. S. de Koning et al., J. Sep. Sci., 27(5–6), 397 (2004).
9. S. de Koning , H.G. Janssen and U.A.T. Brinkman, J. Chromatogr. A, 1058(1–2), 217 (2004).
10. W.C. Quigley, C.G. Fraga and R.E. Synovec, J. Microcolumn Sep., 12(3), 160 (2000).
11. R. Edam et al., J. Chromatogr. A, 1086(1–2), 12 (2005).
12. S. de Koning, H.G. Janssen and U.A.T. Brinkman, LCGC Eur., 19(11), 590 (2006).
13. D. Thiebaut and E. Robert, Analysis, 27(8), 681 (1999).
14. J.M. Levy, J.P. Guzowski and W.E. Huhak, J. High Resolut. Chromatogr., 10(6), 337 (1987).
15. J.M. Levy et al., J. Chromatogr. Sci., 27(7), 341 (1989).
16. Z. Liu et al., Chromatographia, 35(9–12), 567 (1993).
17. A. Venter, P.R. Makgwane and E.R. Rohwer, Anal. Chem., 78(6), 2051 (2006).
18. F. Adam et al., J. Chromatogr. A, 1217(8), 1386 (2010).
19. T. Dutriez et al., Patent FR1002248, 2010.
20. B. Omais et al., J. Chromatogr. A, Submitted.
21. E.J. Guthrie and H.E. Schwartz, J. Chromatogr. Sci., 24, 236 (1986).
22. J. Beens et al., J. Chromatogr. A, 919(1), 127 (2001).
23. J. Beens et al., J. Chromatogr. A, 1086(1–2), 141 (2005).
24. T. Dutriez et al., Energy Fuels, 24, 4430 (2010).
25. A. Venter, E.R. Rohwer and E.R. Laubscher, J. Chromatogr. A, 847(1–2), 309 (1999).
26. R.E. Paproski, J. Cooley and C.A. Lucy, Analyst, 131(3), 422 (2006).
27. W.E. Rudzinski and T.M. Aminabhavi, Energy Fuels, 14(2), 464 (2000).
28. C. West and E. Lesellier, J. Chromatogr. A, 1110(1–2), 200 (2006).
29. C. West and E. Lesellier, J. Chromatogr. A, 1115(1–2), 233 (2006).
30. S. Momchilova and B. Nikolova-Damyanova, J. Sep. Sci., 26(3–4), 261 (2003).
31. I. Francois, A.D. Pereira and P. Sandra, J. Sep. Sci., 33(10), 1504 (2010).
32. P.E. Andersson, M. Demirbuker and L.G. Blomberg, J. Chromatogr., 595(1–2), 301. (1992).
33. E. Lundanes and T. Greibrokk, J. Chromatogr., 349(2), 439 (1985).
34. H. Skaar et al., J. Microcolumn Sep., 2, 222 (1990).
35. T. Dutriez et al., J. Sep. Sci., 33, 1787 (2010).
36. B. Omais et al., J. Chromatogr. A, 218, 3233 (2011).
New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.












