Separations of Intact IgGs Using Non-Porous, 2 m Particle, C18 Presto Column, with a View Towards ADC Analysis
The Application Notebook
Intact and reduced IgGs have been successfully retained and separated using a non-porous column. Potential applications of Presto columns for a single-injection separation of free drug, antibody, and antibody-drug complexes (ADCs) is also discussed.
Introduction
Strategies for chromatographic separation of large proteins typically use wide pore (300 Ã ) particles with C4 alkyl chains. However, this is fundamentally limited by the pore size in that large proteins may experience significant steric hindrance. Another disadvantage is that short chains usually adversely affect resolution of small molecules, such as when a sample contains both large and small molecules (for example, antibody-drug conjugates, ADCs).
One solution to these challenges is our Presto FF-C18 column, which is packed with non-porous, 2 µm, C18 modified silica particles. The advantages of this design are trifold. Firstly, since there are no pores, there is virtually no size limit for molecules that can be injected on the column. Secondly, small molecules can also be retained more efficiently on C18 and produce sharper elution peaks. Thirdly, both of the large and small molecules' separations can occur in a single injection-ideal for those who want to determine fractions of free drug, free antibody, and antibody-drug complex (ADC).
Experimental Conditions
Presto FF-C18 (250 à 4.6 mm length à ID); mobile phases: (A) water + 0.1% trifluoroacetic acid, (B) acetonitrile + 0.1% trifluoroacetic acid; 400 µL/min; 85 °C; sample 2 mg/mL; 2 µL injection volume. Gradients are 0 to 60 min for: 32â43% for IgG1#1, 34â41% for IgG2#1, 33â42% for IgG4#1. UV detection at 220 nm, operating pressure 15 MPa.
Results and Discussion
Chromatograms for intact and reduced IgGs are shown in Figure 1aâc and Figure 1dâf, respectively.
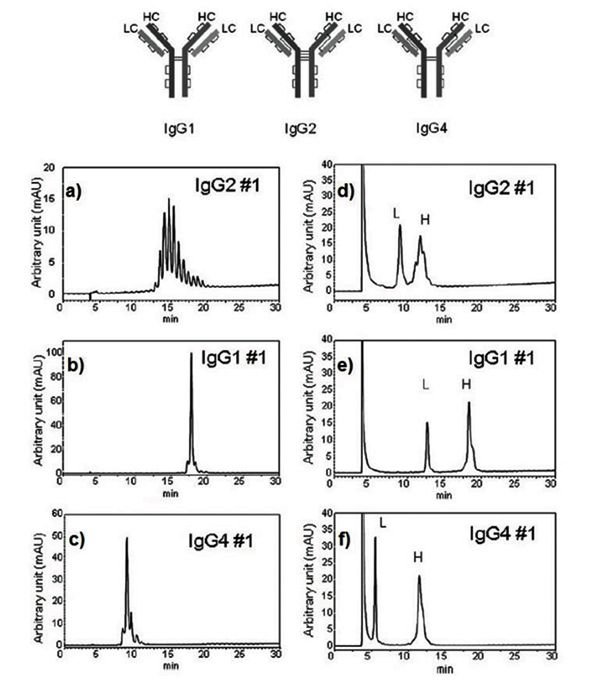
Figure 1: Figure 1 a to c - Intact IgGs, d to f - reduced IgGs.
The IgGs #1 and 4 presented in Figures 1b and 1c show excellent peak shape. The IgG #2 (Figure 1a) exhibits multiple, partially co-eluting peaks indicating multiple isoforms within the IgG #2 subclass. Despite that, it is clear that these proteins can be easily separated using Presto FF-C18 column as their retention times are sufficiently distinctive.
Figure 1dâf presents chromatographic separations of IgGs in their reduced forms. The light chain fraction peaks (L) in all cases, elute earlier compared to intact IgGs. In contrast, heavy chain fraction (H) elute at approximately the same time as intact IgGs indicating their primarily role in intact protein retention time. Further, light chains elute in a single peak, while heavy chains are somewhat asymmetrical and exhibit shoulders. This observation is consistent with the possibility that variations in charge on the chains, and their folding dynamics are likely to exist.
Conclusion
Presto FF-C18 showed excellent separation capabilities for several IgGs. Proteins were eluted with high resolution for both intact proteins and their reduced forms (light and heavy chains), which has not been previously possible on reversed phase columns. The quality of the separation was shown to extend over a wide range of molecular sizes making this column a potentially attractive strategy for many applications, for example, ADCs analysis.

Imtakt USA
1104 NW Overton St., Portland, OR 97209
tel. (888) 456-HPLC, (215) 665-8902, fax (501) 646-3497
www.imtaktusa.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.