Self-Assembled Nanomaterials for Enhanced Chemical Separations
LCGC North America
Nanomaterials offer exciting possibilities for improving separations with techniques such as TLC, SPME, and CE.
This installment focuses on advanced analytical separations that incorporate nanomaterials for improved chemical selection. These new nanomaterials are based on bioinspired and polymeric self-assembled aggregates. Capillary electrophoresis and chromatographic methods were harnessed for the separation of a wide variety of samples. Compelling applications include rapid separations of 100 DNA fragments and separations of enantiomers, aptamers, small molecules, and glycans. The implementation, performance, and fundamental properties of these new media are discussed.
The Pittsburgh Conference of Analytical Chemistry and Applied Spectroscopy (Pittcon), was held March 12–18, 2011, at the World Congress Center in Atlanta, Georgia. The Pittcon 2011 technical program boasted a number of exciting advances in analytical chemistry, including a number of leading edge technologies in the area of separation science. A session sponsored by the American Chemical Society's (ACS) Subdivision of Chromatography and Separations Chemistry on self-assembled nanomaterials for enhanced chemical separations was organized by Professor Lisa Holland of the Department of Chemistry, West Virginia University (Morgantown, West Virginia).
Presentations in this Pittcon session outlined enhanced separations and diverse applications with techniques based on new materials for thin-layer chromatography (TLC), solid-phase microextraction (SPME), and capillary electrophoresis (CE). Some separation media discussed in this session emanated from fabrication techniques such as photolithography and chemical vapor deposition. A new "spin" on fabrication was nanofiber electrospinning. Self-assembly of biomolecules inspired by natural systems was a prominent theme. Generally, these biological selectors were effective for the separation of biopolymers such as DNA, aptamers, and glycans, as well as biologically relevant small molecules. Classical self-assembled surfactant micelles were adapted to improve electrically driven separations.
The purpose of this installment of "Column Watch" is to summarize some of the key techniques presented in this session and discuss future directions that new self-assembled nanomaterials may take in separation science.
Background in Self-Assembled Nanomaterials for Separation Science
Traditional separation media such as chromatographic packing material or open-tubular chromatography columns are fabricated through the covalent modification of a polymeric or inorganic (silica, for example) surface. With new advances in material science, newer strategies arise that result in sophisticated architecture for chemical separations based on molecular self-assembly. These processes result from spontaneous molecular interactions to control the size, shape, or surface characteristics of the assembly, often resulting in what is a highly ordered and unique structure. A benefit of this approach is that reduced chemical modification results in a greatly simplified fabrication protocol of what is a complex final product. Assembled structures may be made from components that are inorganic or organic (or both) or biological in nature. Frequently, such materials originate from or are inspired by biological systems, such as cellular membranes. The resulting supramolecular assembly can occur through a variety of mechanisms such as ionic, hydrophobic, or hydrophilic interactions, as well as adsorption. Although individual interactions may be weak, the summative force from these interactions stabilizes the entire assembly. These molecular assemblies may possess nanoscale features as well as unique properties that macroscale materials often lack.
Nanofabrication: The Patterned Nanotube Forest or the Imprinted Nanofiber?
David Jensen and colleagues from the Department of Chemistry and Biochemistry at Brigham Young University (BYU, Provo, Utah) reported ongoing work in carbon nanotube–templated porous materials. Prototype devices have been constructed with a variety of nanofabrication methods. The methods, as discussed at the Pittcon session (1) and outlined in the literature (2), provide control of both the 3-D microscale shape as well as the porosity of what might be described as a patterned carbon nanotube forest. These materials can be used as high-tech silica-based TLC plates. In the session, Jensen presented several custom-fabricated normal-phase TLC plates that incorporated a herringbone pattern (Figure 1). This pattern facilitated higher separation efficiencies and shorter run times as compared to state-of-the-art commercially available TLC plates. This work was done in the laboratory of Matthew Linford at BYU and was also directed by Robert Davis in the BYU Physics department. It was sponsored by US Synthetic Corp. (Orem, Utah), which intends to commercialize the work.
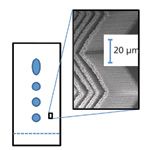
Figure 1: Depiction of thin-layer chromatography based on the herringbone pattern shown in the SEM image in the inset that is achievable with silicon-carbon core-shell nanotubes.
A twist on previously reported ultrathin-layer chromatography (3,4) based on electrospun carbon nanofibers was introduced by Susan Olesik and coworker Joseph Zewe of Ohio State University (Columbus, Ohio). As depicted in Figure 2, nanofibrous stationary phases are produced through electrospinning different polymer solutions from a syringe attached to a high-voltage power supply onto a grounded substrate surface. In TLC, the resulting stationary phase provides superior separation efficiencies to commercial counterparts. Electrospinning also can be used to fabricate unique self-assembled and self-polymerizing molecular imprinted fibers (5). The work presented at Pittcon 2011 (6) centered on a solid, yet ultrathin mat of carbonaceous material imprinted with the chemical warfare agent surrogate dibutyl butyl phosphonate. With simple electro-spinning and pyrolysis, the imprinted material was incorporated into an SPME material and used to extract dibutyl butyl phosphonate from both simple and complex matrices. In both cases, with and without interferences, imprinted SPME media demonstrated enhanced selectivity for the template molecule, extracting more warfare simulant than nonimprinted SPME fibers. Molecularly imprinted carbon is both highly chemically and thermally stable, which are also advantages over traditional molecularly imprinted polymers.

Figure 2: Schematic of the straightforward process for electrospinning nanofibers adapted from reference 2.
Bioinspired Self-assembled Materials: If Nature Will Not Be Replicated in the Laboratory, Can It Be Plagiarized?
Separation materials derived or modeled from those discovered in biological sources offer a wide variety of functionality as separation material. Guanosine gels, for example, reversibly self-assemble as a function of temperature, compound concentration, solution composition, and pH (7). These materials have previously been harnessed polymorphic to separate single-stranded DNA in capillary gel electrokinetic chromatography based on the DNA sequence. In a new application reported in the session by Yingying Dong of Rensselaer Polytechnic Institute (Troy, New York) (8), the unique morphology of this medium was harnessed for the separation of chiral enantiomers (9). The self-assembled materials form columnar "G-wires" stabilized by π-π interactions, leading to helical, asymmetric structures. When integrated in CE, the authors were able to separate enantiomers of molecular weight <300 Da. The material is inexpensive and simple to prepare, and the method provides reproducible migration times. Baseline resolution was achieved within 10 min and 16 min for enantiomers of tryptophan and BNPA, respectively (see Figure 3). Interestingly, not all enantiomers could be separated, leading to structural studies of the interactions between the G-gels and chiral compounds that will provide a basis for modifications of the G-gels to achieve chiral separations for a wider range of compounds.

Figure 3: Capillary electrophoresis separations of tryptophan enantiomers (top) and BNPA enantiomers (bottom) using guanosine-5'-monophosphate run buffer (0.05 M GMP and 0.05 M KCl in 25 mM potassium phosphate buffer, pH 7.0).
Another bioinspired self-assembled material used for CE separations was presented by the Holland laboratory at West Virginia University. The phospholipids dimyristoylphosphatidylcholine (DMPC) and dihexanoylphosphatidylcholine (DHPC) can self-assemble into different morphologies and offer a number of advantages including a thermally responsive viscosity. At room temperature, preparations of these phospholipids have a viscosity similar to that of water. However, at temperatures a few degrees higher preparations exhibit a significantly increased viscosity (10). An important consequence of this thermal transition is that the material can be easily loaded into the capillary at temperatures below 24 °C, facilitating a gel-like separation in CE with a greatly simplified column preparation method. Following separation of the analytes at the elevated temperature, the capillary temperature can be dropped, and the phospholipid can be expelled and replaced as needed. One application is the separation of single- and double-stranded DNA. Lisa Holland and coworkers (11) demonstrated the utility of phospholipid additives to efficiently separate solutions of double-stranded DNA that vary in length by only a few base pairs. As shown in Figure 4, the method was also used to identify conformational changes of single-stranded DNA aptamers and to probe binding of an aptamer to its selected target. Another benefit of the high viscosity of this thermally responsive phospholipid media is that other selection agents can be incorporated into the material and immobilized in the separation capillary without any covalent methods of binding. In addition to conformational change, the material has been loaded with aptamer target to retard the aptamer migration through the media, thus demonstrating molecular affinity.

Figure 4: Example of the use of a phospholipid capillary separation integrated with thrombin to capture thrombin-binding aptamer. The two traces show the separation of a single-stranded DNA aptamer that is a 15-mer specific for thrombin separated in the presence (lower trace) and absence (upper trace) of the thrombin plug.
This medium also proved ideal to separate glycans (12,13), which are branched oligosaccharides relevant to numerous biological studies and applications. When glycans are derivatized with the fluorescent label 8-aminopyrene-1,3,6-trisulfonic acid (APTS), they may be detected using laser-induced fluorescence at low limits of detection and at a labeling stoichiometry of 1:1, facilitating quantification. Additionally, the APTS label imparts negative charge to the glycan molecule. When the glycan is neutrally charged, for example following desialylation, the derivatization enables glycan separation based on hydrodynamic volume. The added viscosity provided by phospholipid media enhances the separation of these glycans, even resolving glycans with the same monomer composition and sequence, but differing only in a single terminal linkage. When integrated in a 25-µm i.d. capillary, theoretical plate counts in excess of 600,000 are obtained. Stephanie Archer-Hartmann (14) and colleagues from West Virginia University presented an application of this separation method by assessing complex mixtures of glycans derived from glycoprotein standards and from biological samples through the incorporation of proteins such as lectins or enzymes directly into the capillary. Examples included the lectin concanavalin A, which binds strongly to high mannose glycans, as well as exoglycosidases that remove terminal mannose and galactose from the glycan. All of these separations were accomplished without the need for covalent modification of either the capillary or the protein. Archer-Hartmann demonstrated the use of this method to quickly survey glycoproteins derived from the MCF7 immortalized human breast cancer tumor cell line with the intention of efficiently separating, but also discerning, structural characteristics of the derived glycans.
A Return to the Micelle: Unique Separation Mechanism with Self-Assembled Surfactants
For more than 25 years, the role of micelles as a vehicle to support partitioning in electrokinetic separations has been established (15), and yet, the role of micelles in electrophoresis has expanded again. James W. Schneider of Carnegie Mellon University (Pittsburgh, Pennsylvania) reported on a novel, gel-free method to electrophoretically separate DNA oligomers using nonionic surfactant micelles (16). The oligos are tagged by hybridization of an end-alkylated peptide-nucleic acid (PNA) surfactant to their 5' end (17) or to both 5' and 3' ends (18). These peptide nucleic acid–tagged oligos are then separated in CE using a running buffer containing approximately 3 vol% of nonionic surfactant micelles. During the run, micelles bind the peptide nucleic acid tags to retard the electrophoretic mobility of the oligomers in a length-dependent fashion. Because gels are not used, the run times are 10–100 times faster than capillary gel electrophoresis and are compatible with commercially available CE instrumentation. The key to success of the method is a rapid fluctuation of micelle size during the run, so that highly uniform drags are experienced by all oligos in the sample. A model was presented predicting run times for various applications, including DNA sequencing, identification of STR loci, and mRNA profiling.
Finally, in covering the development of self-assembled materials to improve chemical separations, Charles Lucy and colleague Bob Stanley of the University of Alberta (Edmonton, AB, Canada) addressed the disassembly of self-assembled media (19). Micellar electrokinetic chromatography (MEKC) is a powerful separation technique, but surfactants strongly suppress ionization in electrospray mass spectrometry (MS) making MEKC–MS challenging. The Canadian workers explored the model acid-labile surfactant, sodium 4-[(2-methyl-2-undecyl-1,3-dioxolan-4-yl) methoxy]-1-propane sulfonate (ALS), as a media for micelle electrokinetic capillary chromatography separations (Figure 5). The optimal conditions for bulk synthesis and separation were characterized. Efficiencies of greater than 100,000 plates and migration time reproducibilities comparable to sodium dodecyl sulfate were achieved in ALS-MEKC. After separation, the ALS can be hydrolyzed off-line (t1/2 = 48 min) to nonsurface active products compatible with electrospray ionization.
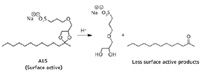
Figure 5: Acid hydrolysis of the surfactant ALS.
The Future of Self-Assembly Nanomaterials: Could Be in Discovery of Nanomaterials That Provide an Added Function to the Analyte Separation
Additional talks during this session at Pittcon 2011 included presentations on novel nanomaterials to enhance methods of detection and optical methods of analysis. At Louisiana State University (Baton Rouge, Louisiana), such innovative nanomaterials could ultimately be the secret ingredient hidden in the group of uniform materials based on organic salts (GUMBOS), according to Sergio L. de Rooy (20). These nanomaterials with thiacarbocyanine compounds originate from a group of uniform materials based on organic salts that are prepared via an ion-exchange reaction. For these nanomaterials distinct morphologies exhibited spectral properties based on Foster resonance energy transfer as well as organic dye aggregation. Jiao Chen and colleagues (21) of the University of North Dakota (Grand Forks, North Dakota) reported the production of metallic nanostructures to enhance fluorescence intensity with applications in a number of different bioanalytical studies. From Korea University (Seoul, South Korea), Man Bock Gu and colleagues (22) conveyed the role of the affinity ratio in colorimetric nanoparticle-based aptasensors. The utility of the approach was demonstrated by the selective detection of five different small molecule–based targets. Nancy Xu of Old Dominion University (Norfolk, Virginia), gave a presentation on nanoparticles as "nano rulers" (23) and as probes of size-dependent transport kinetics of receptors in cells (24). Can new materials hold potential to sense and separate? Perhaps the answer will be revealed at Pittcon 2012.
References
(1) D. Jensen, A. Dadson, M. Linford, M. Vail, R. Vanfleet, R. Wyman, R. Davis, and S. Kanyal, Pittcon 2011, Paper 115-7, March 13, 2011.
(2) J. Song, D.S. Jensen, D.N. Hutchison, B. Turner, T. Wood, A. Dadson, M.A. Vail, M.R. Linford, R.R. Vanfleet, and R.C. Davis, Advanced Functional Materials 21(6), 1132–1139 (2011).
(3) J.E. Clark and S.V. Olesik, Journal of Chromatography A 1217(27), 4655–4662 (2010).
(4) J.E. Clark and S.V. Olesik, Anal. Chem. 81(10), 4121–4129 (2009).
(5) J.W. Zewe, J.K. Steach, and S.V. Olesik, Anal. Chem. 82(12), 5341–5348 (2010).
(6) S.V. Olesik and J. Zewe, Pittcon 2011, Paper 815-1, March 15, 2011.
(7) Y. Yu, D. Nakamura, K. DeBoyace, A.W. Neisius, and L.B. McGown, The Journal of Physical Chemistry B, 112(4), 1130–1134 (2008).
(8) Y. Dong and L. McGown, Pittcon 2011, Paper 815-3, March 15, 2011.
(9) W.S. Case, K.D. Glinert, S. LaBarge, and L.B. McGown, Electrophoresis 28(17), 3008–3016 (2007).
(10) T. Pappas and L. Holland, Sensors and Actuators B: Chemical 128, 427–434 (2008).
(11) L.A. Holland, B. Durney, and S. Archer-Hartmann, Pittcon 2011, Paper 115-6, March 13, 2011.
(12) R.J. Luo, S.A. Archer-Hartmann, and L.A. Holland, Anal. Chem. 82, 1228–1233 (2010).
(13) S.A. Archer-Hartmann, L.M. Sargent, D.T. Lowry, and L.A. Holland, Anal. Chem. 83(7), 2740–2047 (2011).
(14) S.A. Archer-Hartmann, L. Holland, and X. Wu, Pittcon 2011, Paper 115-1, March 13, 2011,
(15) S. Terabe, K. Otsuka, K. Ichikawa, A. Tsuchiya, and T. Ando, Anal. Chem. 56, 111–13 (1984).
(16) J. W. Schneider, A. Holmen, M. Fahrenkopf, and S. Istivan, Pittcon 2011, Paper 815-2, March 15, 2011.
(17) S.T. Grosser, J.M. Savard, and J.W. Schneider, Anal. Chem. 79(24), 9513–9519 (2007).
(18) J.M. Savard, S.T. Grosser, and J.W. Schneider, Electrophoresis 29(13), 2779–2789 (2008).
(19) C.A. Lucy and B. Stanley, Pittcon 2011, Paper 115-8, March 13, 2011.
(20) S.L. de Rooy, B. El-Zahab, I. Warner, M. Li, and S. Das, Pittcon 2011, paper 115-3, March 13, 2011.
(21) J. Chen, N. Fahrudding, W. Thompson, and Y. Jin, Pittcon 2011, Paper 115-2, March 13, 2011.
(22) M.B. Gu, J.H. Kim, S.J. Lee, and Y.S. Kim, Pittcon 2011, Paper 115-4, March 13, 2011.
(23) N. Xu, P. Nallathamby, and T. Huang, Pittcon 201, Paper 815-4, March 15, 2011.
(24) N. Xu, K. Lee, and P. Nallathamby, Pittcon 2011, Paper 115-5, March 13, 2011.
Ronald E. Majors "Column Watch" Editor Ronald E. Majors is Senior Scientist, Columns and Supplies Division, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column via e-mail to lcgcedit@lcgcmag.com.

Ronald E. Majors
Stephanie Archer-Hartmann is a Ph.D. candidate in the Chemistry Graduate program at West Virginia University. Her research encompasses bioanalytical chemistry and separation science in the Holland laboratory. As the recipient of a United States Pharmacopeia fellowship, Stephanie is refining capillary electrophoresis methods to characterize protein glycosylation.

Stephanie A. Archer-Hartmann
Lisa Holland currently holds a faculty position in the C. Eugene Bennett Department of Chemistry at West Virginia University, specializing in bioanalytical separations. Dr. Holland is the recipient of a National Science Foundation Faculty Early Career Development award, serves on the Executive Board of the American Chemical Society Subdivision of Chromatography and Separations Chemistry, and has numerous publications in the field of separations chemistry.

Lisa A. Holland

Perspectives in Hydrophobic Interaction Temperature- Responsive Liquid Chromatography (TRLC)
TRLC can obtain separations similar to those of reversed-phase LC while using only water as the mobile phase.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





