HPLC Systems and Components Introduced at Pittcon 2011: A Brief Review
LCGC North America
This year, many HPLC systems focused on facilitating process development, method transfer, and biological analysis.
The Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy (Pittcon) is a showcase for the latest advances in laboratory instrumentation and technology, attracting all of the major high performance liquid chromatography (HPLC) vendors and a total of more than 1000 exhibiting companies from around the world. Pittcon is a great place for vendors to introduce, and for scientists to see, touch, evaluate, compare, and hear all about the latest in HPLC technology. This installment of "Innovations in HPLC" highlights some of the new HPLC and related technology introduced at this year's conference.
Because Pittcon attracts upwards of 20,000 attendees from industry, academia, and government from more than 90 countries worldwide, the conference provides a great opportunity for vendors to expose new high performance liquid chromatography (HPLC) products to both new and existing customers. With this fact in mind, many HPLC vendors have for years coordinated their new product development cycle around Pittcon, introducing new technology at the conference for the first time. Although the proliferation of smaller, specialized conferences has altered this approach somewhat in recent years (for example, many new mass spectrometry [MS] product introductions are now made at the American Society for Mass Spectrometry annual conference), Pittcon still remains a must-attend meeting to see the latest and greatest in HPLC and related technology. For the attendees, the conference provides a venue to evaluate the latest instrumentation, compare vendors, participate in product demonstrations, and speak with technical staff to resolve problems or investigate potential applications.
At this year's Pittcon, several vendors introduced new systems and components, as well as product line extensions. In this installment of "Innovations in HPLC," I'll review some of the new HPLC instrument technology shown at the conference; Table I lists the introductions reviewed in this column. (New column and sample preparation technology is reviewed in LCGC by Ron Majors in his "Column Watch" column.)
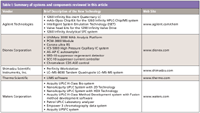
Table I: Summary of systems and components reviewed in this article
Biologics Analysis
Sometimes referred to as biotech drugs, biopharmaceuticals, or biotherapeutics, biologicals are protein- or peptide-based therapeutics (also included are nucleic acids, whole cells, monoclonal antibodies, viral particles, and vaccines) derived from either genetically altered bacteria or fungi or from blood and blood plasma products. Recombinant proteins and monoclonal antibodies (mAbs) are examples of two of the most prevalent biologics; follow-on biologics, or biogenerics, is another area garnering increased attention. Many pharmaceutical and biotechnology companies are continuing to look for new ways to improve the formulation and delivery of biologic drugs. Because this area is the fastest growing market segment in the pharmaceutical industry, several HPLC instrument vendors have begun to focus on this growing market segment, as evidenced by Pittcon 2011 product introductions. Many Pittcon 2011 instrument introductions for biologics analysis were extensions of existing instrument platforms (ultrahigh-pressure liquid chromatography [UHPLC] remains a growing technology platform for many vendors), while some were based on new instrument technology.
Waters (Milford, Massachusetts) introduced three offerings in this area: the Acquity UPLC H-Class Bio system, the NanoAcquity UPLC system with 2D Technology, and the NanoAcquity UPLC system with HDX Technology. Waters showcased its next-generation UHPLC instrument called the Acquity UPLC H-Class system at Pittcon in 2010. The Acquity UPLC H-Class Bio System advances macromolecule analysis through multiple-mode chromatographic capability, automated quaternary solvent blending, and the use of corrosion-resistant inert materials. Designed for the analysis of proteins, peptides, nucleic acids, and glycans, it has the flexibility to perform four chromatographic modes on a single system: reversed-phase chromatography, ion-exchange chromatography, size-exclusion chromatography (SEC), and hydrophilic interaction chromatography (HILIC). A key feature of the system is the Auto-Blend Plus feature, which ensures that mobile phase compositions and gradients are automatically blended to achieve the optimum retention and selectivity requirements. The Auto-Blend Plus feature automatically manages pH and ionic strength requirements for the mobile phase by calculating the proportions of buffer stocks required for desired conditions. Computation can be based on known pK values or on an empirical calibration table, making any possible buffer combination available. Users also can create their own library of combinations. Figure 1 shows an example of the H-Class system using autoblend for the analysis of a protein mixture.
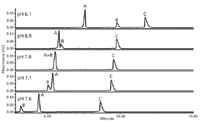
Figure 1: A mixture of proteins separated by cation-exchange chromatography using the Waters Acquity UPLC H-Class Bio system. The Auto-Blend Plus feature was used to automatically find the optimum concentration of mobile phase modifier.
The NanoAcquity UPLC System with 2D Technology uses two-dimensional (2D) UHPLC for better chromatographic resolution of complex proteomic samples by using a dual reversed-phase approach, which results in better protein identifications, quantification, and sequence coverage. The 2D system uses reversed phase at pH 10 in the first dimension, followed by reversed phase at pH 2 in the second dimension for results that far exceed those of conventional ion-exchange methodologies.
The NanoAcquity UPLC system with HDX Technology uses UHPLC separations and high-resolution MS for the analysis of biotherapeutics and proteins, and for the routine determination of changes in protein conformation, including drug binding, product stability, and protein interactions. By carefully controlling temperature-sensitive parts of the HDX workflow, conformational changes are not missed. The separation takes place within the cooled environment with the added advantage of sub-2-µm particles for enhanced chromatographic resolution. Maintaining temperatures at 0 ± 1 °C means that back-exchange is kept to an absolute minimum. Once the hydrogen deuterium exchange reaction has been chemically quenched, the HDX Manager directs the sample to a pepsin column for peptic digestion, or directly into the MS system for global analysis. The sample is then trapped for a rapid UHPLC analytical separation.
Agilent Technologies (Santa Clara, California) introduced two systems with applications in biologics, the 1260 Infinity Bio-inert Quaternary LC and a new mAb-Glyco Chip Kit for the company's 1260 Infinity HPLC-Chip/MS system. The 1260 Infinity Bio-inert Quaternary LC system is based on the 1200 Infinity Series LC platform. The system is 100% inert with completely metal-free capillary surfaces in the sample flow path and no stainless steel (for example, no nickel-, chromium-, cobalt-, or manganese-containing alloys or other alloys that are problematic for biological samples) in the mobile phase flow path. It has a wide pH range (1–13) and can easily tolerate 2 M salt and 8 M urea. Options include a bioinert fraction collector, fluorescence detection, and column- and solvent-selection capabilities.
The Agilent 1260 Infinity HPLC-Chip/MS System is a microfluidic chip-based technology for nanospray LC–MS introduced in 2005. It combines nanoflow HPLC columns, connecting capillaries, and an electrospray emitter into a re-useable, credit card-size device allowing nano LC–MS to be accessible to scientists without the troublesome setup involving the microvalves, fittings, and capillary tubing of conventional nano LC. With the Pittcon 2011 introduction of the new mAb-Glyco Chip kit, Agilent now offers 12 versions of HPLC-Chips for multiple applications. The mAb-Glyco Chip includes an enzyme reactor for on-chip deglycosylation of mAbs, enrichment and analytical columns for concentration and separation of the cleaved N-glycans, and an electrospray tip. The system includes predefined optimized methods for HPLC-Chip/MS analysis, automated comprehensive data processing tools (including an accurate mass glycan database for ease of characterization), reporting templates, and all reagents and chemicals required.
Dionex (Sunnyvale, California) introduced the UltiMate 3000 mAb Analysis Platform, which is a fully biocompatible system that features a dual ternary gradient pump design in a 2-in-1 system concept. The system's autosampler includes an integrated fraction collector with the ability to automatically reinject samples into a second dimension. Using the system, cell culture fluid samples containing antibodies can be injected onto a protein A column for antibody recovery and the purified antibody samples can be injected onto an SEC column for aggregate analysis and then injected onto an ion-exchange column for charge variant analysis.
Historically, conductivity monitoring has been used in the HPLC separation of biologics to accurately follow gradient formation. More recently, on-line pH monitoring has gained interest in the emerging application of pH gradient ion-exchange chromatography of monoclonal antibodies and charge variants. An introduction by Dionex featured the new PCM-3000 module that allows on-line pH and conductivity monitoring in these types of HPLC applications. The module mounts to the UltiMate 3000 UV absorbance detector and features flow cells for analytical LC.
Shimadzu Scientific Instruments (Columbia, Maryland) introduced the Perfinity Workstation. This system, which was developed in partnership with Perfinity Biosystems (West Lafayette, Indiana), uses novel and proprietary separation technologies to automate sample preparation and analysis of serum proteins. The system prepares peptides ready for LC–MS analysis directly from serum within 10 min. The system consists of five columns; each column performs one step of the sample preparation process: affinity selection, buffer exchange, digestion, desalting, and reversed-phase separation. Automated integration of these steps makes the overall analysis up to 50 times faster than conventional approaches. Standard immunoassays have been the dominant method of performing routine serum protein analyses for the last 60 years for good reason; however, proteins that vary in biological activity are impossible to discriminate using standard antibody approaches. Although LC–MS is capable of resolving these differences, proteins must be extracted or samples fractionated before analysis. The system's solution is to pair the selectivity of antibodies with the resolving power of LC–MS. Figure 2 illustrates a comparison of a traditional protein digest to that obtained using the system.
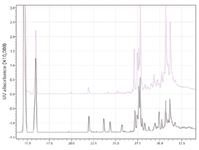
Figure 2: Comparison reversed-phase chromatography of a protein digest obtained by traditional (top) and Perfinity Workstationâderived (bottom) digests.
Method Development and Transfer
HPLC and UHPLC systems intended for automating method development and facilitating method transfer have also been introduced at recent Pittcons. This year, new systems and technology were introduced by at least two vendors. Waters formally introduced a system, previewed at last year's Pittcon in Orlando, that combines the Acquity UPLC H-Class system with Fusion method development software (S-Matrix Corporation, Eureka, California). Using quality-by-design (QbD) principles, this combination, along with the introduction of Waters' Method Development Chemistry kits, helps to develop more robust analytical methods in a fraction of the time it takes using traditional HPLC, taking automated method development to entirely new levels of efficiency and productivity, and reducing costs.
Instrument-to-instrument method transfer is an important topic for many laboratories, where HPLC and UHPLC methods are transferred between different departments or locations with different LC instruments. Agilent Technologies chose Pittcon 2011 to introduce Intelligent System Emulation Technology (ISET) for the 1290 Infinity LC system. This feature harnesses the performance of the 1290 Infinity LC to emulate other systems for the seamless transfer of methods between LC systems, regardless of brand. ISET is designed to accelerate and facilitate the transfer of analytical LC methods between laboratories using different HPLC or UHPLC instruments. Users can take full advantage of the speed and resolution of the newest UHPLC approaches while still running their legacy HPLC methods. This dual-capability means that users can safely invest for the future without disrupting analytical workflows, even in regulated environments. LC method development laboratories can be more productive by taking advantage of faster UHPLC techniques and transferring methods to HPLC by emulating that instrument.
Agilent Technologies also introduced two new valve head kits for the 1290 Infinity Valve Drive, the latest addition to the 1290 Infinity LC system. It is compatible with all currently available Agilent Quick Change Valve heads and Agilent 1220, 1260, and 1290 Infinity LC systems, as well as with older 1100 and 1200 Series LC systems, facilitating a multitude of column- and solvent-selection configurations for method development.
With these two new kits, Agilent now has 12 different valve head kits for flexibility in HPLC and UHPLC automation. The first kit is a 1200-bar, six-column selector valve head kit for use with the 1290 Infinity thermostated column compartment that supports up to four columns, a waste line, and a bypass line. Used together with the new 1290 Infinity Valve Drive, as many as six columns are supported (the limitation inside the column compartment is because of available presolvent heat exchangers). Together with this latest addition to the valve portfolio, Agilent customers can decide between two-, four-, six-, or eight-column selection configurations in an LC system by a simple mouse-click, depending on the valve head used. The second kit is a 12-position selection bioinert valve head kit. Typically, this kit will be used with the 1290 Infinity Valve Drive for solvent selection or fractionation at low pressures (up to 200 bar).
HPLC Detectors
Charged aerosol detection (CAD) was first introduced commercially in 2004 and is based on the combination of HPLC with electrical aerosol technology. CAD is highly sensitive, provides a consistent response, and has a broad dynamic range, offering real advantages to researchers and analysts for a variety of applications, particularly when analyzing compounds lacking UV chromophores.
Introduced this year, the Corona ultra RS is the newest Dionex CAD system. This version adds features that enhance the usability and extend the applications for the Corona ultra detector. The RS version includes an on-board switching valve that allows the detector to be used independently of other detectors, without the need for system reconfiguration. The same valve can be used to divert high salt from samples. An optional variable ratio flow splitter is available to make it easier to use CAD in parallel with MS without compromising resolution, and a new data processing algorithm makes it possible to optimize the data output for specific applications. The detector is fully compatible with both HPLC and UHPLC methods, and when used with the company's UltiMate 3000 dual gradient system, the detector provides consistent response independent of the molecule's chemistry for nonvolatile analytes with or without a chromophore.
Triple-quadrupole MS is the method of choice for accurate quantification and confirmation of trace-level analytes in complex matrices, from the detection of drugs and metabolites in biological specimens to environmental contaminants and pesticides in food. Keeping pace with the sensitivity and speed demanded by today's HPLC and UHPLC separations, Shimadzu introduced the LCMS-8030 system, which couples the power of a triple-quadrupole MS system that claims industry-leading fast multiple reaction monitoring (MRM) transitions (500/s), fast polarity switching, (15 m/s), and scan speeds of 15,000 u/s.
Ion Chromatography
Dionex expanded its ion chromatography (IC) offerings with several Pittcon 2011 introductions. The ICS-5000 High Pressure Capillary IC system allows analysts to increase throughput and lower costs by conducting fast IC separations, or to achieve higher separation efficiencies through the use of longer columns. The new capillary version of the system can operate continuously at pressures as high as 5000 psi. Also introduced was the AS-AP IC autosampler for capillary IC and fast IC applications. The autosampler has options for automatic dilution and an integrated sample conductivity and pH accessory; concentration and matrix removal applications, along with low carryover and a contamination-free flow path, make it ideal for ultratrace ion analysis. It also supports dual IC systems, fraction collection, and sample preparation. The autosampler offers fast cycle times and high sample capacity, from 81 samples with 10 mL vials to 1152 samples using microwell plates, supporting fast IC and providing the needed capacity for high-throughput laboratories. The chemically inert PEEK flow path withstands pH extremes and protects sensitive samples from metal contamination. Figure 3 shows separations obtained using the ICS-5000 High Pressure Capillary IC system.

Figure 3: Fast determination of a 19-anion standard using a Dionex High Pressure Capillary IC system. An IonSwift MAX100G, MAX100, 0.25-mm column operated at 35 °C and a KOH gradient was used. The injection volume was 0.4 µL, and detection was by suppressed conductivity. Peaks: 1 = fluoride, 2 = acetate, 3 = formate, 4 = butyrate, 5 = galacturonate, 6 = chloride, 7 = nitrite, 8 = bromide, 9 = nitrate, 10 = carbonate, 11 = sulfate, 12 = oxalate, 13 = tungstate, 14 = phosphate, 15 = chromate, 16 = citrate, 17 = isocitrate, 18 = cis-aconitate, and 19 = trans-aconitate.
In addition, Dionex introduced the SRD-10 suppressor regenerant detector and the SCC-10 suppressor current controller. The suppressor regenerant detector is a stand-alone device that monitors liquid flow to a suppressor and disables the eluent pump if flow is restricted or stops, preventing irreversible damage to the suppressor.
The suppressor current controller is an external adapter designed for use with legacy instruments that offer only four settings for electrolytic suppressor current. The controller's output current can be set in 12 discrete steps from 10 to 250 mA, thus increasing suppressor life while simultaneously reducing noise. It also provides greater flexibility in selecting optimal operating conditions and suppressor current settings for many IC applications. Because the controller connects the suppressor's power supply to the suppressor, and because it is powered from the existing suppressor power supply, it is not necessary to change the system configuration in the control software for use.
Process Development
To address the needs of process development chemists and QbD, Waters introduced the Patrol UPLC Laboratory analyzer, an integrated system solution designed and engineered to perform laboratory and pilot-scale online reaction monitoring in the process development laboratory in a fully automated and compliant-ready manner. Based on the company's UPLC platform, it is designed to detect and quantify the components of complex reaction mixtures and fully characterize a candidate molecule by LC, LC–MS, and LC–MS-MS before moving it into process development. The analyzer enables process development scientists to directly analyze the progress of reactions online by Real-Time LC or LC–MS to generate quantitative results, including low-level reaction components and trace process impurities. A hallmark of the system is its dilution accuracy, dilution linearity, and dilution range. The technology behind this performance is the instrument's process sample manager (PSM), which automatically extracts an online sample from a reactor or slipstream, and performs sample preparation and sample injection — a process that once required the time and full attention of an analytical technician. In addition to online samples, the PSM can also accept and store up to 32 barcode-labeled vials, which can be a combination of different standards, controls, and even at-line samples.
Software
Several new software introductions were made at Pittcon this year, including laboratory information management systems (LIMS) software, chromatography data systems (CDS), and extensions of existing software to extend control capabilities.
Thermo Fisher Scientific (Waltham, Massachusetts) launched a significant release of the company's flagship laboratory data management software, which is designed for large-scale process and industrial laboratories and is developed to facilitate compliance with ISO and GLP. Thermo also showcased LIMS software for water and environmental applications, designed to help laboratories keep pace with the rapidly changing regulatory environment, including the Safe Drinking Water Act and the EPA's Drinking Water Infrastructure Needs Survey and Assessment.
Waters debuted the next version of its popular Empower product, the Empower 3 chromatography data system, which is a scalable, enterprise-wide CDS platform that can be rapidly deployed and fit easily into existing corporate infrastructure. The software is designed to address the needs of technology managers for data management standardization, including reduced training efforts and support resources, improved internal communication and information exchange, and the facilitation of more-consistent regulatory compliance through easier software validation and streamlined review and sign off of results. In addition, the software offers a variety of usability and workflow enhancements that help streamline users' daily workflow and efficiency. The software features improved navigation between the review and reporting applications and also allows users to create sample sets after acquisition. This feature enables more flexibility in processing injections that were acquired individually and also helps with multi-stage and delayed-release dissolution testing. Additional calculation capabilities allow the ability to determine current signal-to-noise requirements, according to regional pharmacopeias in the United States, European Union, and Japan.
Another unique feature of the CDS software is the capture of Acquity UPLC column usage information, including information such as column name, serial number, the number of injections on that column, maximum pressure, and maximum temperature. This information is tracked for each sample set for automatic and accurate documentation purposes; it is also tracked on a rolling basis so users can obtain column trending information and extrapolate and predict lifetime information.
In the area of sample preparation, Dionex announced Chromeleon CDS control of its ASE 350 automated solvent extraction system. The control capabilities feature control of chromatographic systems, creation of secure audit trails, and hard copy reports not available from standard front panel controls.
Supercritical Fluid Chromatography
Supercritical fluid chromatography (SFC) has been commercially available since around 1982. SFC can be differentiated from gas chromatography (GC) and HPLC by the use of a supercritical fluid as the mobile phase.
SFC has several advantages over GC and HPLC, including rapid separations without the use of organic solvents, reduced use of organic additive chemicals, and the use of carbon dioxide collected as a by-product of other chemical reactions (or collected directly from the atmosphere), contributing no new chemicals to the environment. In addition, SFC separations can be done faster than HPLC separations because the diffusion of solutes in supercritical fluids is about 10 times greater than that in liquids (and about three times less than in gases). The lower diffusion results in a decrease in resistance to mass transfer in the column and allows for fast high resolution separations. Compared with GC, capillary SFC can provide high-resolution chromatography at much lower temperatures. This allows fast analysis of thermolabile compounds. Many vendors have recognized these advantages and have begun to offer SFC instruments on existing HPLC and UHPLC platforms.
The new Agilent 1260 Infinity Analytical SFC system uses the Aurora SFC Fusion A5 (Aurora SFC Systems, Inc, Redwood City, California) module to achieve high sensitivity to facilitate low-level impurity analysis as well as enantiomeric excess determination, in chiral compound separations. It is available as a complete system or as an upgrade to existing Agilent 1100 and 1200 Series or 1260 Infinity LC systems. Four orders-of-magnitude of dynamic range provide accurate quantitation of 0.01%-level impurities with high precision, HPLC-like sensitivity, and UHPLC speed using UHPLC columns. The system is fully compatible with the manufacturer's CDS software. Figure 4 illustrates the separation of a chiral compound with two chiral centers using the Agilent 1260 Infinity SFC LC system. All four stereoisomers were baseline separated isocratically.
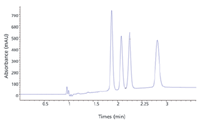
Figure 4: Isocratic SFC separation of four stereoisomers of a chiral pharmaceutical compound with two chiral centers using an Agilent 1260 Infinity SFC LC system.
Waters also introduced an SFC system, the Acquity UPSFC system, which is built on the company's UPLC sub-2-µm particle chemistry platform. The UltraPerformance supercritical fluid chromatography (UPSFC) system is designed to incorporate all of the advantages of SFC, in addition to superior separation, resolution, and speed afforded by the sub-2-µm particle chemistry platform. When used in chiral and achiral compound applications, the sensitivity and separation capability of the system enables users to rapidly confirm the purity of compounds and to determine enantiomeric excess. Compared to traditional analytical SFC, the sub-2-µm particle system can run more samples per day with shorter cycle times, faster re-equilibration, and higher resolution. The system is fully compatible with the company's Empower and MassLynx informatics software and with the company's Viridis SFC Columns that are specifically designed for SFC.
Conclusions
The information in this review is based partially on manufacturers' responses to a preconference questionnaire mailed in late 2010. I used the information received in the questionnaires that were returned, as well as information from personal communication, to try to make this review as comprehensive as possible. But keep in mind that, because of the sheer size of the conference and the fact that some manufacturers did not respond to the questionnaire or do not release pre-show information, this report cannot be considered an exhaustive listing of all new products introduced in Atlanta. I'm sure there were a lot of additional noteworthy items at Pittcon, but time, space, and the scope of this column prevent me from covering them all in detail. I'm bound to have missed a few items or details, and I apologize for any omissions. In 2012, Pittcon will return to Orlando, March 11–16. I hope to see you there!
Michael Swartz "Innovations in HPLC" Editor Michael E. Swartz, Ph.D., is Principal Scientist in Analytical Development at Ariad Pharmaceuticals, Cambridge,Massachusetts, and a member of LCGC's editorial advisory board. Direct correspondence about this column to "Innovations in HPLC," at lcgcedit@lcgcmag.com.

Michael Swartz

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).













