Selection of Columns for GCxGC Analysis of Essential Oils
LCGC Europe
Demonstrating the suitability of a new wax stationary phase for GCxGC analysis of essential oils
Introduction
Multidimensional separations are performed by combining single analytical separation columns in such a way to greatly enhance peak capacity for the separation of complex multi-component samples. Comprehensive multidimensional gas chromatography (GC×GC) employs largely independent first- and second-dimension separation mechanisms and typically generates peak capacity of the order of several thousand, making it a highly appropriate technology for the separation and analysis of complex multicomponent samples such as essential oils.
A substantial majority of GC×GC publications for essential oil analysis in the periodical literature have used a "non-polar" column in the first dimension and employed a "polar" column in the second dimension.1 In practice this almost always translates to use of a 100% polydimethylsiloxane or 5% diphenyl 95% dimethyl polysiloxane stationary phase in the first-dimension combined with a 50% diphenyl 50% dimethyl polysiloxane or a polyethylene glycol (wax) second-dimension column, although there are notable departures from this convention, including applications reversing the order of "polarity",2 providing class-type separation of citrus oil components and those using cyclodextrin derivative stationary phases for enantioselective analysis.3–6 Even in the case where a conventional non-polar/polar column ensemble is used, the mechanism of retention for solutes in the second dimension depends on volatility and polarity, but using an appropriate temperature programme cancels out the influence of volatility on retention in the second dimension column.7 Thus, GC×GC separations are temperature-programmed to maximize differences in separation mechanisms in the two dimensions.
However, because both columns are commonly installed in the same oven, the temperature stability of one of the separation columns typically imparts an upper temperature limit on the separation system. Applications that use 50% diphenyl 50% dimethyl polysiloxane second dimension columns are benefited in terms of high-temperature stability compared with those applications employing wax columns, but more suitable selectivity of wax columns for polar essential oil components was shown many years ago and wax stationary phases are almost universally preferred.8
Recently, we received a 5 m × 100 μm i.d. MEGA-WAX HT high-temperature wax column with a stationary phase film thickness of 0.10 μm (Mega, Legnano, Italy) and have been using this column for our GC×GC work involving essential oils analysis. The wax second dimension column used in the current investigation has more than 9000 N/m tested in isothermal mode and an upper column oven limit of 300 °C. Results from the GC×GC analysis of kunzea essential oil obtained by steam distillation of aerial parts of Kunzea ambigua are reported here.
Results
Kunzea ambigua is a shrub native to the Eastern parts of Australia. As an effective killer of bacteria, kunzea essential oil has numerous traditional folk medicine uses and researchers have recently developed a kunzea oil formulation for veterinary use.9 Kunzea oil is rich in sesquiterpene alcohols and as a consequence one-dimensional GC lacks the resolving power to provide adequate separation of the complex oil. In the present investigation a GC×GC column set comprising a 30 m × 250 μm i.d. Rxi-5Sil MS column with a stationary phase film thickness of 0.25 μm (Restek, Bellefonte, Pennsylvania, USA) was installed as the first dimension separation column and the second dimension column was a 1 m × 100 μm i.d. MEGA-WAX HT column with a stationary phase film thickness of 0.10 μm. The primary goal of this investigation was to determine the suitability of the MEGA-WAX HT column as a second-dimension column for essential oil analysis, in particular for providing adequate separation of the sesquiterpene alcohols in kunzea oil. To date, we have not attempted comprehensive peak identification of the essential oil components using GC×GC–MS but we anticipate that these results will be reported in time.
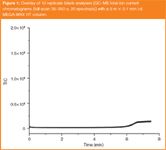
Figure 1
Prior to removing a 1.0 m length from the MEGA-WAX HT column, a series of fast GC–MS analyses were performed using the 5 m column as received. All analyses were performed using a Shimadzu GC–MS-QP2010-Plus (Shimadzu Oceania, Melbourne, Australia). A fast temperature programmed gradient from 40 °C to 300 °C in 6.5 min (40 °C/min) was employed for these analyses and the oven was held at the maximum temperature for 1 min at the end of the temperature programme. The carrier gas (He) was delivered at a constant pressure of 36 psi. Figure 1 shows a zoomed-in overlay of 10 replicate blank analyses [GC–MS total ion current chromatograms (full-scan 35–350 u; 20 spectra/s)] that were performed to assess the level of stationary phase bleed. Even using a reasonably aggressive rapid temperature programme rate up to 300 °C these results are highly satisfactory. The average baseline response (n = 10) at the maximum oven temperature (6.5–7.5 min) was less than 7.5× the average baseline response recorded between 2 and 5 min.
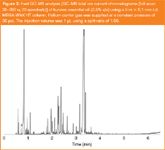
Figure 2
Fast GC–MS analysis of the kunzea essential oil was also performed using this configuration and a typical chromatogram is shown in Figure 2. Note the maximum intensity of the small peak marked # in Figure 2, which has a low relative peak intensity of 1.09%, is more than 19× the average baseline response between 2 and 5 min. To compare the general selectivity of the MEGA-WAX HT column, a fast GC–MS chromatogram of kunzea essential oil using a 20 m × 100 μm i.d. Rtx-WAX column is shown in Figure 3. Temperature effects as well as differences in the stationary phase chemistry each influence the absolute retention order so an attempt to overlay these chromatograms would be pointless. The MEGA-WAX HT column is also 75% shorter than the Rtx-WAX column, so the resolution using the latter column is understandably better. Nonetheless, elution of the monoterpene and sesquiterpene hydrocarbons, followed by the monoterpene and sesquiterpene alcohols is in general congruence between the two columns.

Figure 3
GC×GC analyses were performed using an Agilent 6890 GC (Agilent technologies, Australia) equipped with a custom dual-jet cryogenic (CO2) modulation system based on the design of Beens et al.10 and a flame ionization detector. A temperature programme of 60 °C to 300 °C in 48 min (5 °C/min) was employed for all analyses. A typical GC×GC chromatogram of kunzea essential oil is shown in Figure 4. Following application of a three second modulation period there is excellent utilization of the two-dimensional separation space albeit with a substantial amount of wrap-around of the more polar solutes. Such a degree of wrap-around is not uncommon for essential oils analysis by GC×GC and this is generally acceptable provided that the wrapped-around components don't interfere with those belonging to the next modulation. It would be possible to re-optimize the separation using a four or five second modulation period to reduce the observed wrap-around, but this option was avoided in the current study primarily because the wrap-around was not too extreme and we chose to obey the three to four slice per peak modulation criterion for maintaining first dimension separation integrity.11
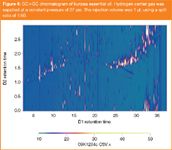
Figure 4
The separation performance of the MEGA-WAX HT second-dimension column is further highlighted in Figure 5, where an extracted peak slice is presented. The peak capacity of the second dimension column is estimated to be approximately 10 using the conditions described above and we conclude that this new stationary phase makes an excellent choice for fast GC–MS or GC×GC analysis of essential oils.
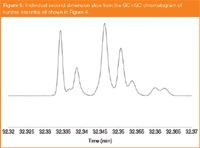
Figure 5
Acknowledgements
This work was supported by the Australian research Council's Discovery funding scheme (project number DP0771893). The authors wish to thank Dr Stefano Galli (Mega) for providing MEGA-WAX HT columns, Dr Christian Narcowicz (University of Tasmania) for supplying the kunzea essential oil sample. The generous support from Restek Corporation is gratefully acknowledged.
Philip Marriott is professor of Separation Science at RMIT University, and deputy director of ACROSS. His interests are in high resolution separations, particularly GC, GC×GC and capillary electrophoresis, with mass spectrometry amongst other detection techniques.
Paul Morrison is research technical associate in the Australian Centre for Research on Separation Science (ACROSS) group at RMIT. He implements high resolution separation strategies and develops strategic instrumental capabilities within ACROSS.
Samuel Poynter is a PhD student in ACROSS at University of Tasmania (UTAS).
Robert Shellie is senior lecturer at UTAS. He leads a research group in ACROSS (UTAS) that focuses on development and application of hyphenated techniques in chromatography to solve complex separation problems.
References
1. R.A. Shellie, Comprehensive Anal. Chem., 55, 189–213 (2009).
2. L. Mondello et al., Flavour Fragr. J., 20(2), 136–140 (2005).
3. R. Shellie, P. Marriott and C. Cornwell, J. Sep. Sci., 24(10–11), 823-830 (2001).
4. R. Shellie and P.J. Marriott, Anal. Chem., 74(20), 5426–5430 (2002).
5. M. Wang et al., J. Chromatogr. A, 1112(1–2), 361–368 (2006).
6. M. Junge et al., Anal. Chem., 79(12), 4448–4454 (2007).
7. J.B. Phillips and J. Beens, J. Chromatogr. A, 856(1–2), 331–347 (1999).
8. P. Marriott et al., Flavour Fragr. J., 15(4), 225–239 (2001).
9. J. Thomas et al., Vet. Rec., 164, 619–623 (2009).
10. J. Beens et al., J. Chromatogr. A, 919(1), 127–132 (2001).
11. P. Schoenmakers, P. Marriott and J. Beens, LCGC Eur., 16(6), 335–339 (2003).
Coupling Matters: Robert Shellie
Does coupling matter to me because I was taught to think that way? After all, I obtained my formal training in separation science at the RMIT node of the Australian Centre for Research on Separation Science (ACROSS): the birthplace of GC×GC in Australia and the home of cryogenic modulation. In fact I liken my fascination with hyphenated chromatographic techniques to television. At home, Australians are rapidly turning to HDTV — more pixels supposedly enhance the viewing experience. Likewise when one sees a really high-resolution multidimensional separation happening in real-time, it is difficult to imagine why one would attempt to analyse a complex sample any other way.
I have just celebrated my 10-year anniversary of working with multidimensional chromatography. In my current role in ACROSS at University of Tasmania I am lucky enough to be able to share the development of coupled techniques with the next generation of separation scientists. I was very pleased to have been asked to continue Robert Smits' contribution to LCGC Europe and I look forward to sharing the latest coupling matters developed by likeminded researchers from all parts of the globe in this column. Robert Shellie
LCGC Europe would like to thank Robert Smits for the success of "Coupling Matters" under his tenure and his invaluable support with the magazine. Direct Correspondence about this column to "Coupling Matters", LCGC Europe. Poplar House, Park West, Sealand Road, Chester CH1 4RN, UK. E-mail: amatheson@advanstar.com
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.














