Secrets to Successfully Translating and Transferring HPLC Methods
When transferring HPLC methods between instruments, many parameters need to be considered. Knowledge of underlying principles and a few key equations will help you.
Very often we have a need to transfer or translate an existing high performance liquid chromatography (HPLC) method to a different instrument or to gain speed by altering our column geometry, stationary phase particle size, or particle morphology.
To do this successfully we need to understand the underlying principles and important instrument aspects that govern this translation or transfer. No matter if you are transferring to a smaller column geometry with the same particle size or to a column using smaller particles, certain simple relationships hold that can help us to geometrically scale to the optimum eluent flow, pressure, sample volume, or the expected efficiency we might obtain.
Further, there are simple formulas to help us transfer the gradient profile to maintain selectivity and retention order.
There may also be times when we need to transfer an existing method from one instrument to another, without any change other than the actual instrument used, the instrument manufacturer, or the laboratory in which it is situated. Many of the considerations highlighted below will be pertinent to this situation also.
It should be noted that in all situations below, the nature of the stationary phase chemistry does not change between the original and translated method.
Flow rate between columns with different diameter and particle size can be scaled using the following simple relationship.
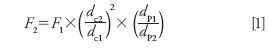
where F is flow rate in milliliters per minute, dc is column diameter in millimeters, dp is particle diameter in micrometers, 1 is the original value, and 2 is the translated (new) value.
Injection volume can be translated using equation 2:
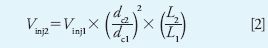
where Vinj is the injection volume in microliters and L is the column length in millimeters. All other terms are the same as in equation 1.
The expected pressure from the new column can be approximated using equation 3:
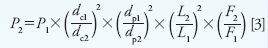
where p is the pressure in bar or pounds per square inch and all other terms are the same as in equations 1 and 2.
The expected change in efficiency can be calculated using equation 4:

where N is the plate count and all other terms are the same as in the other equations.
Please bear in mind that the measurement of plate count in gradient HPLC is virtually meaningless — although for comparative purposes it will give an idea of the expected improvement in the peak width and therefore the chromatographic performance.
One of the most important parameters in scaling methods is the gradient time (tg) for each gradient segment. By keeping the gradient composition the same at each point, altering the gradient time will alter the slope of the gradient, which is the important thing in terms of preserving retention order and selectivity. There are a host of more complex relationships that are featured in the CHROMacademy webcast and tutorial, but as pragmatic chromatographers we prefer simple rules of thumb that can be performed quickly and easily within the laboratory and will give us results that are fit for purpose.
The time for each gradient segment can be calculated using equations 5 and 6:
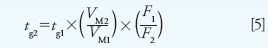
where tg is the gradient time in minutes, VM is the column interstitial volume (volume of mobile phase inside the column) in microliters, and other terms are the same as previously mentioned.

Solving equation 6 for a 50 mm × 2.1 mm column would result in a mobile-phase volume of 110 μL, for example.
Of course, when translating gradient methods between instruments, one would need to consider the gradient dwell volume (time) for each system to compensate properly for these differences. This discussion is outside the scope of this short article but full details can be found in the accompanying CHROMacademy Essential Guide.
So, just to test out your calculator, we have translated a method and shown the original and final values in Table I — see if you can match these values and prove to yourself that you can easily translate method variables in HPLC!
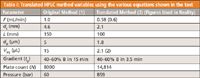
Table I: Translated HPLC method variables using the various equations shown in the text

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.