Robust and Repeatable Nanoparticle Drug Delivery Characterization with FFF–MALS–DLS
Nanoparticles hold enormous potential for targeted, well-controlled drug delivery. Extensive characterization of nanoscale drug-delivery vehicles is essential to ensure their efficacy and reproducibility. While various methods are available to characterize nanoparticle size, including dynamic light scattering (DLS), electron microscopy, and nanoparticle tracking analysis, field-flow fractionation–multi-angle light scattering (FFF–MALS) is one of the most versatile techniques to determine size, structure, and other properties. This article highlights a study demonstrating the benefits of FFF–MALS–DLS for the characterization of nanolipid particles.
Daniel Some, Wyatt Technology Corporation, Santa Barbara, California, USA.
Photo Credit: PASIEK/ Getty Images

Nanoparticles hold enormous potential for targeted, well-controlled drug delivery. Extensive characterization of nanoscale drug-delivery vehicles is essential to ensure their efficacy and reproducibility. While various methods are available to characterize nanoparticle size, including dynamic light scattering (DLS), electron microscopy, and nanoparticle tracking analysis, field-flow fractionation–multi-angle light scattering (FFF–MALS) is one of the most versatile techniques to determine size, structure, and other properties. This article highlights a study demonstrating the benefits of FFF–MALS–DLS for the characterization of nanolipid particles.
Nano-drug-delivery systems (nanoDDSs), increasingly recognized as the basis for the next generation of drug delivery, have already proven significant benefits over traditional formulations. However, nanoDDS are inherently more complex in structure and composition than common drug forms. In order to realize their promise, extensive characterization is required throughout research, development, and production. Techniques for the characterization of nanoparticle size are plentiful. Recent studies have shown that FFF–MALS1,2 is a highly beneficial technique for nanoDDS characterization and provides well-resolved and accurate size distributions as well as structure and composition, which overcome many of the limitations of other technologies. This article presents case studies illustrating the characterization of liposomes and nanolipid complexes by FFF–MALS, demonstrating the technique to be an easily adaptable, yet powerful, characterization tool.
Nanoparticles for Drug Delivery
The limitations of many conventional pharmaceutical preparations, including solutions, suspensions and emulsions, often include low availability, intolerance, instability, and a lack of sustained effect. One of the most active research directions to overcome these limitations is nanomedicine, which applies nanotechnology to highly specific medical interventions for prevention, diagnosis, and treatment of diseases. The surge in nanomedical research during the past few decades is now translating into considerable commercialization efforts around the globe.
Nanoparticles are attractive because nanoparticle fabrications can be precisely controlled, allowing their size, shape, surface charge, stability, and other physical characteristics to be modified to influence particle behaviour in vivo. These properties are exploited to enhance uptake and bioavailability, efficacy of transfection through the cellular membrane, and other aspects of pharmacokinetics. Nanoparticles also exhibit a large surface-to-volume ratio, allowing the surface coating to be functionalized with ligands for highly specific applications such as targeting of biomarkers. These features result in a concomitant reduction in the quantity of the drug required and dosage toxicity, which ultimately enables the safe delivery of toxic therapeutic drugs and protection of non-target tissues and cells from severe side- effects.
Characterization of Nanoparticle Drug Delivery Vehicles
The biological response of nanomedicines depends on specific physico-chemical characteristics. Even small variations in physico-chemical characteristics of a system can have a significant impact on nanomedicine performance. Many different nanoDDSs have been described, including liposomes, hydrogels, silver-coated nanolipids, dendrimers, and colloidal drug carriers, each entailing a different mix of physical and chemical properties. Therefore, in order to maximize the potential of nanoparticles for drug delivery, accurate and detailed characterization is critical in the course of development and production. Successful development of a nanoparticle drug delivery mechanism requires thoughtful selection of the most appropriate orthogonal and complementary characterization techniques.
For the characterization of nanoparticle size and aggregation, several methods are available and routinely used, including dynamic light scattering (DLS), electron microscopy (EM), and nanoparticle tracking analysis (NTA). DLS3 is one of the most common techniques for particle size measurements, providing low-resolution and semi-quantitative size distributions, albeit with good statistical sampling. DLS is widely considered to be user-friendly and fast, yielding relatively consistent results relating to average size and aggregates. In a high-throughout plate reader configuration,4 DLS is particularly beneficial for formulation screening and production QC. However, DLS on its own does not provide particularly good resolution, quantitative distributions, structural, or compositional information.
On the other hand, EM is well-suited for detailed studies of nanoparticle size and shape. It provides a deeper insight into the structure and morphology of individual particles via electron micrograph images of very high resolution. However, EM is not suitable for routine, statistically valid characterization and requires lengthy and operator-intensive analysis.
NTA makes it possible to analyze nanoparticle population in terms of both size and number. NTA provides a more quantitative size distribution than DLS; however, despite its advantages, the technique has comparable size resolution and inferior sampling statistics.
Field-flow fractionation (FFF5) for the separation and characterization of nanoparticles has increased in recent years. FFF provides high-resolution separation of particles as a function of their hydrodynamic size from one nanometre up to several micrometres, the perfect range for use in determining accurate and well-resolved nanoparticle size distributions. Moreover, when the technique is coupled with on-line spectroscopic, multi-angle light scattering (MALS6) and dynamic light scattering detectors, a powerful system is created for versatile, robust characterization of molar mass and size distributions as well as structure and composition. MALS determines molar mass, size (Rg, rms radius), and particle number density. DLS determines hydrodynamic size (Rh, hydrodynamic radius). The shape parameter ρ = Rg / Rh indicates conformation or shape. In addition, spectroscopic techniques such as UV–vis, refractive index, or fluorescence are helpful in determining concentration and composition. FFF–MALS (including on-line DLS) is amenable to the analysis of simple to complex solutions that may include small molecules, macromolecules, colloids, and nanoparticles.
FFF–MALS can provide high-resolution analysis of size, medium-resolution analysis of conformation, and insight into drug or ligand loading into the nanoparticle’s interior or onto its surface, with good statistical sampling.7,8 It can separate and characterize nanoDDSs components such as free drug (payload), ligands (targeting mechanism), or unassembled/partially-assembled polymer or protein (nanoparticle building blocks). It is suitable for robust, repeatable, and routine analyses. In addition to sequential MALS, DLS, and spectroscopic analyses, FFF offers on-line analysis of electrophoretic mobility for determination of well-resolved size/zeta potential distributions.9 This article presents a small sample of the potential applications of FFF–MALS–DLS for nanoDDS characterization.
Liposome Characterization by FFF–MALS–DLS
Liposomes are commonly used as carriers for delivery of drug compounds or RNA-based therapeutics. Drugs may be encapsulated in the liposome’s core, incorporated into the lipid bilayer, or attached to the outer surface. During development and formulation, the size, structure, and drug loading of the liposomes must be assessed. While low-resolution DLS size measurements are often expedient, high resolution analysis of size distributions and structure by FFF–MALS is essential for well-characterized products. In the following case study,10 the analytical results of two liposome samples, one empty and one filled with a therapeutic are reported, using FFF combined with MALS and on-line DLS.
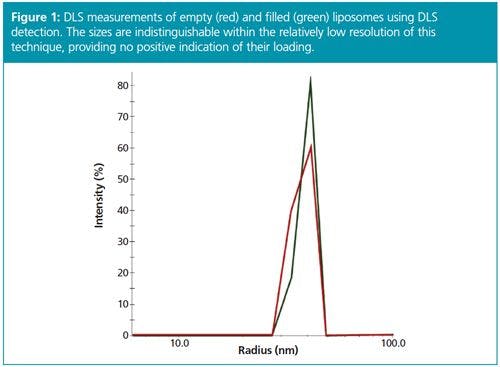
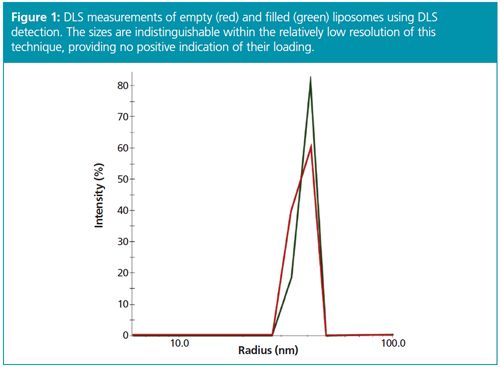
Standard (unfractionated) DLS measurements of filled and empty liposomes are shown in Figure 1. Since filling the liposome core does not change its external dimensions, and Rh is basically a measure of the envelope of the particles, both filled and empty liposomes exhibit nearly identical hydrodynamic radii. Therefore standard DLS is not suitable for identifying drug encapsulation in these samples.
Experimental
For fractionated FFF–MALS–DLS measurements, the Eclipse FFF system was followed by a DAWN HELEOS MALS detector incorporating an embedded on-line DLS module (WyattQELS), all from Wyatt Technology. The size range accessible to accurate DLS analysis generally depends on flow rate and detection angle; here the DLS scattering angle was approximately 143° to extend the Rh measurement from 0.5 nm up to 300 nm. The FFF method was designed with the aid of FFF simulation and optimization software (Wyatt). ASTRA software for MALS analysis (Wyatt Technology) also provided absolute number densities of nanoparticles that have a known, real refractive index.
Results
Both Rg and Rh are plotted against elution time in Figure 2. The results from duplicate runs demonstrate excellent reproducibility of the FFF–MALS–DLS method.
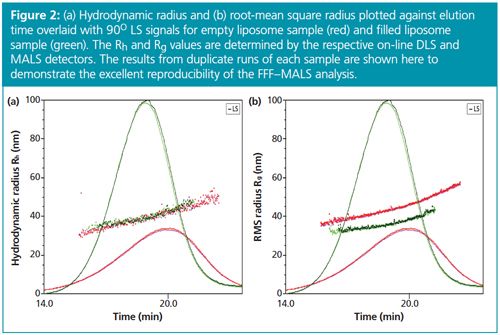
Figure 2 shows that the Rh values for empty and filled liposomes are nearly identical, bearing out the initial batch DLS data albeit with much higher resolution. However, Rg values for these two liposomes do not overlay, which indicates that both liposomes have different degrees of encapsulation.
Root-mean square radii Rg were then plotted against hydrodynamic radii Rh for these two liposomes (Figure 3[a]). The slope of Rg versus Rh plot yields the shape factor, ρ, which is indicative of the structure of the liposomes. For the empty liposome sample ρ equalled 1.0 – consistent with a spherical shell structure. For the filled liposome sample, however, ρ = 0.77, which is in excellent agreement with that of a solid sphere structure of uniform density. The shape factor may also be used to determine the aspect ratio of solid rods or ellipsoids. Absolute number densities of nanoparticles are shown in Figure 3(b).
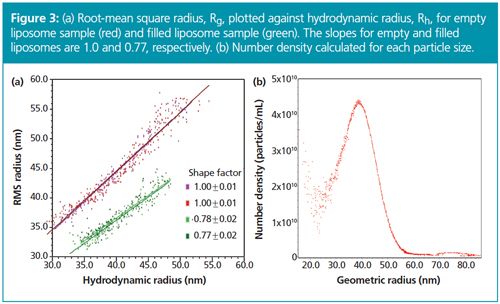
Figure 2 shows that the Rh values for empty and filled liposomes are nearly identical, bearing out the initial batch DLS data albeit with much higher resolution. However, Rg values for these two liposomes do not overlay, which indicates that both liposomes have different degrees of encapsulation.
Silver-Nanolipid Complex Characterization by FFF–MALS
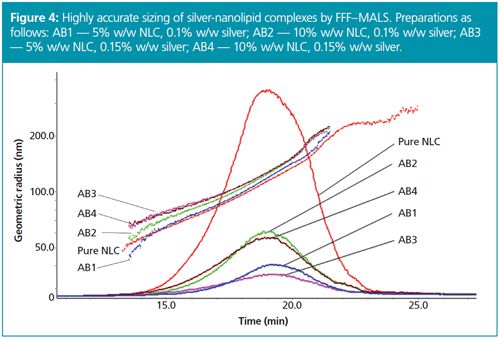
In another case study, FFF–MALS was used to analyze nanolipid complexes (NLC) as carriers functionalized with anti-microbial microsilver particles.11 Functionalized nanoparticles were prepared by incubating two different NLC dispersions (5.0% and 10.0% w/w) in two different microsilver dispersions (0.1% and 0.15% w/w) for a total of four different preparations, to assess silver loading onto the NLC. The resulting dispersions could not be differentiated by DLS or zeta potential. However, FFF–MALS showed distinct and highly repeatable size differences, as depicted in Figure 4. Saturation of the NLCs clearly occurs when exposed to 0.15% w/w microsilver dispersions, but not 0.1%, leading to size changes on the order of 10–15 nm in radius. With repeatability better than 1% in size and accurate, quantitative, high-resolution distributions thanks to true hydrodynamic size separation prior to on-line light scattering measurements, FFF–MALS demonstrates the effect of the four different preparative conditions on the complex formation and helps determine the optimal production conditions.
Conclusions
The surge in nanomedical research for drug delivery mechanisms has highlighted the importance of accurate and detailed characterization techniques for the development and clinical use of therapeutic nanoparticles, to ensure efficacy, safety, and reproducibility. A number of characterization techniques including DLS, EM, and NTA are routinely used, but do not always provide the requisite level of detail and information.
FFF–MALS–DLS is a more versatile option than the common sizing techniques, and we have not even begun to scratch the surface in terms of spectroscopic analyses or on-line zeta potential measurements. The case studies demonstrate that for liposomes and related carrier vehicles, FFF–MALS–DLS is an easily adaptable and powerful characterization tool to obtain information on particle size, size distribution, particle count, as well as structure. Already utilized extensively to address environmental and synthetic polymer nanoparticles, exosomes, and protein aggregates, further adoption of this technique by the nanoDDS community will enhance R&D efforts to optimize physico-chemical properties of nanoparticles in the service of human health.
References
1. S. Podzimek, Light Scattering, Size-exclusion Chromatography and Asymmetric Flow Field Flow Fractionation (John Wiley & Sons, Hoboken, NJ, USA, 2012).
2. P.J. Wyatt, Journal of Colloid and Interface Science197, 9–20 (1998).
3. See www.wyatt.com/Theory/DLS for a brief introduction to DLS
4. D. Some and R. Burge, The Future of Bioprocessing BioPharm International supplement, s2–s8, 23–30 (2015) http://read.findpharma.com/i/592446-biopharm-october-ebook-bioprocessing
5. See www.wyatt.com/Theory/FFF for an introduction to asymmetrical-flow field-flow fractionation
6. See www.wyatt.com/Theory/MALS for a brief introduction to multi-angle light scattering
7. A. Hinna, F. Steiniger, S. Hupfeld, M. Brandl, and J. Kuntsche, Anal. and Bioanal. Chem. 406(30), 7827–7839 (2014).
8. J. Kuntsche, C. Decker, and A. Fahr, J. Separation Sci. 35(15), 1993–2001 (2012).
9. S. Trainoff and D. Some, Chromatography Today [QA: please could you provide any more info such as vol, issue, page numbers for this reference.]
10. M. Chen and V. Lin, “Using FFF-MALS-QELS To Measure Liposome Size Distribution and Conformation”, paper presented at the 2011 International Light Scattering Colloquium, Santa Barbara, CA, USA.
11. C.M. Keck and K. Schwabe, J. Biomed. Nanotech. 5(4), 428–436 (2009) doi: 10.1166/jbn.2009.1053.
Daniel Some is Principal Scientist and Director of Marketing at Wyatt Technology Corporation, where he has spent the last 11 years in various roles including R&D, software development, product management, and marketing. Previously Dr. Some was involved in the development of light-scattering based patterned wafer inspection tools for the semiconductor industry.

Retaining Talent in Field-Flow Fractionation: An Initiative
The authors present their motivation for establishing the Young Scientists of FFF (YSFFF) initiative within the FFF community.
Developments in Field‑Flow Fractionation Coupled to Light Scattering
December 8th 2020Field-flow fractionation (FFF) coupled to light scattering is a powerful method to separate and characterize nanoparticles, proteins, and polymers from a few nanometres to a few micrometres. The technique is one of the few that can cover the full size range of nanomaterials and provide high-resolution size distributions and additional characterization. New developments in FFF enhance performance and productivity.
Field-Flow Fractionation: Virtual Optimization for Versatile Separation Methods
August 8th 2017Flow-field flow fractionation (flow-FFF) offers highly versatile separations for the analysis of complex fluids, covering a size range of macromolecules and particles from 1 nm to 10,000 nm. However, flow-FFF is often perceived as a difficult technique to learn because of the multiple parameters available for adjustment. Recent advances in software for simulating flow-FFF overcome this obstacle, enabling the virtual optimization of flow-FFF methods and opening up the power of flow-FFF separations to non-experts. An added benefit is the ability to easily analyze particle size distributions by elution time from first principles.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)