Preview of Topics at HPLC 2016, Part 2: New Materials for UHPLC Analysis of Monoclonal Antibody Drugs and Antibody–Drug Conjugates
The Column
This is the second in a four-part series of articles exploring topics that will be addressed at the HPLC 2016 conference in San Francisco, USA, from 19–24 June.
Mary J. Wirth, Alexis Huckabee, and Jonathan Yasosky, Purdue University, West Lafayette, Indiana, USA.
Photo Credit: Aaron Foster/Getty Images

This is the second in a four-part series of articles exploring topics that will be addressed at the HPLC 2016 conference in San Francisco, USA, from 19–24 June.
The separation of intact proteins plays many roles in drug discovery and development: identification of drug targets, screening for drug candidates, quality control, and drug authentication. Various separation techniques are used, from immunoprecipitation to study a single protein of interest, through various types of column chromatography for detecting a handful of proteins at once, all the way to proteomics for studying hundreds to thousands of proteins. What all of these techniques and applications have in common is that the power of protein separations is limited by the fact that proteins are large, slowly diffusing, sticky molecules.
There is a demand for even higher performance. One critical need for better chromatography is in the development of antibody–drug conjugates (ADCs). Only a few ADCs have been approved by the US Food and Drug Administration (FDA), but many ADCs are under development and in all phases of clinical trials. An ADC directs a toxic small-molecule drug to cancer cells by covalently binding the toxic drug to a monoclonal antibody through a labile bond.1 The intention is that the labile bond breaks after the ADC is inside the cancer cell, but not before, to release the toxin where it is needed. ADCs have multiple binding sites, and each species potentially has different efficacy and toxicology. Therefore, it is necessary to determine to how many of these binding sites the drug has been attached. Hydrophobic interaction chromatography (HIC) uses columns packed with nonpolar polymer beads to separate ADCs based on how many drug molecules are conjugated to a given antibody (the drug–antibody ratio, or DAR).2 The bands are typically too broad to fully separate all of the species, however, and some ADC candidates are too hydrophobic for current HIC columns. Decades of research and development (R&D) in the chromatography industry have been devoted to reversedâphase liquid chromatography (LC) because this mode of LC is so widely used for small-molecule drugs and for quality control of protein drugs. Creating better bonded phases for HIC, however, is a challenge for silica-based columns because HIC uses a fully aqueous mobile phase and neutral pH.
The bonded phase is a pervasive limitation to efficiency in monoclonal antibody separations. Another problem with monoclonal antibody separations is that silica particles with an average pore diameter of 300 Å do not allow full access to the surface area. The limited pore size also impedes intraparticle diffusion. Advances in the chromatographic materials need to consider all of these issues.
Bonded Phases with Less Protein Adhesion
There are two approaches to reducing silanol activity in HIC columns that our group has considered. One is selfâassembled monolayers for reversedâphase separations, illustrated in Figure 1(a). These monolayers form a dense, two-dimensional net over the surface to improve reversed-phase LC separations of proteins.3 Since coulombic interactions are long-range, any defects in the column produce the familiar tailing of mixed-mode chromatography techniques, which is mitigated by low pH. The other approach, surface-confined atom-transfer radical polymerization, alleviates the coulombic interactions by growing a dense layer of tethered polymer chains that is thicker than the Debye length, as illustrated in Figure 1(b), reducing longârange attractions between proteins and silica.4 Nonporous polymer particles with tethered polymers as bonded phases5 have been commercially available for some time. Despite their small surface area, these particles have been valuable for monoclonal antibody separations, including ion-exchange chromatography and HIC, as mentioned earlier. Our group found that the bonded phase depicted in Figure 1(b) gives excellent hydrophilic interaction liquid chromatography (HILIC), resolving the glycosylation states of ribonuclease B and providing partial resolution of their isomers.6 We are currently applying HILIC to monoclonal antibodies to decrease analysis time for probing glycosylation. We are also investigating whether the combination of self-assembly and
tethered polymer chains on silica can result in a better HIC bonded phase for ADC analysis.
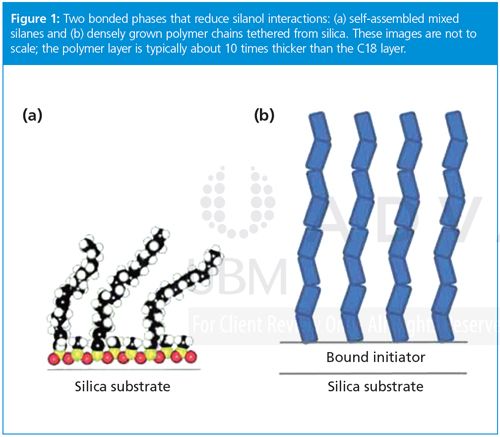
Morphologies to Decrease Diffusion Distance and Increase Accessible Surface Area
The most salient commercial advance in LC over the last 15 years has been the introduction of chromatographic particles that decrease the diffusion distances for mass transport. These include fully porous sub-2-µm particles,7 core–shell particles,8 and monolithic columns.9 Porous materials, regardless of morphology, will eventually approach the limit where the smaller particle size (or smaller shell thickness) approaches the diameter of the larger colloids needed to produce wider pore sizes.10 Sphere-on-sphere particles, which are core–shell particles with a single layer of large colloids,11 embody this idea. As this limit is approached for fully porous particles, it makes sense to consider using the colloids themselves as the packing material.
We have studied capillaries packed with 0.5-µm nonporous silica particles with C4 bonded phases, packed in capillaries. The accessible surface area for large proteins is quite high, falling in between those of core–shell particles and fully porous particles, each for a 300-Å pore size.12 The back pressure is alleviated by the presence of slip flow for reversedâphase LC,13 enabling column lengths of at least 10 cm for commercial high-pressure chromatography instruments. Colloidal particles pack more tightly than standard particles, obstructing the axial diffusion about twofold more. This tighter packing gives rise to a higher velocity inside the column, which makes the optimal volume flow rate slower, further alleviating back pressure. Uniform packing results in negligible eddy dispersion. With no intraparticle diffusion, lower obstructed diffusion, and lower eddy dispersion, all three terms in the van Deemter equation are thus smaller. Barring effects from strong adsorption of proteins, the van Deemter equation predicts submicrometre plate height for proteins.
We observed submicrometre plate heights for protein separations using 0.5âµm particles.12,13,15 Plate heights were as small as 15 nm and the plate number was in excess of 106 for a 2-min separation of proteins.13 In this case, self-assembly was used to produce a mixed C4–C1 bonded phase, injection was done by diffusing the protein into the head of the column to avoid contact between metal parts and protein, and on-column detection was used. For moreâroutine applications, Figure 2 shows an example separation where a commercial autoâinjector was used, with the same bonded phase and on-column detection. An IgG4 monoclonal antibody drug and its two aggregates were resolved well in a short 1-cm separation length at room temperature, whereas a 5-cm long column of fully porous particles did not resolve the three species.
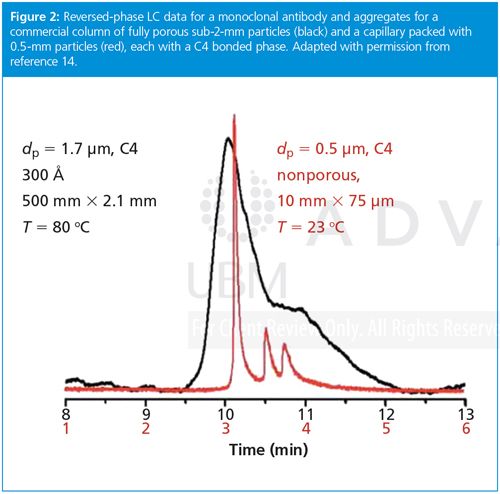
HIC columns will likely enjoy a boost in flow rate from slip flow when used with submicrometre particles. The next goals are to extend the performance seen in Figure 2 to 2.1-mm i.d. stainless steel columns for reversed-phase LC, and combine this advance with a new polymeric bonded phase on silica for HIC of ADCs.
References
- P. Polakis, Pharmacol. Rev. 68, 3–19 (2016).
- M. Rodriguez-Aller, D. Guillarme, A. Beck, and S. Fekete, J. Pharm. Biomed. Anal. 118, 393–403 (2016).
- M.J. Wirth, R.W.P. Fairbank, and H.O. Fatunmbi, Science275, 44–47 (1997).
- X.Y. Huang and M.J. Wirth, Anal. Chem.69, 4577–4580 (1997).
- M. Weitzhandler, D. Farnan, J. Horvath, J.S. Rohrer, R.W. Slingsby, N. Avdalovic, and C. Pohl, J. Chromatogr. A828, 365–372 (1998).
- Z. Zhang, Z. Wu, and M.J. Wirth, J. Chromatogr. A1301, 156–161 (2013).
- J.E. MacNair, K.C. Lewis, and J.W. Jorgenson, Anal. Chem.69, 983–989 (1997).
- J.J. Kirkland, Anal. Chem.64, 1239–1245 (1992).
- N. Ishizuka, H. Minakuchi, K. Nakanishi, N. Soga, and N. Tanaka, J. Chromatogr. A797, 133–137 (1998).
- X.Y. Huang, R.S. McLean, and M. Zheng, Anal. Chem.77, 6225–6228 (2005).
- A. Ahmed, H. Ritchie, P. Myers, and H. Zhang, Adv. Mater.24, 6042 (2012).
- B.J. Rogers and M.J. Wirth, J. Phys. Chem. A117, 6244–6249 (2013).
- B. Wei, B.J. Rogers, and M.J. Wirth, J. Am. Chem. Soc.134, 10780–10782 (2012).
- B.J. Rogers, R.E. Birdsall, Z. Wu, and M.J. Wirth, Anal. Chem.85, 6820–6825 (2013).
- B.A. Rogers, Z. Wu, B.C. Wei, X.M. Zhang, X. Cao, O. Alabi, and M.J. Wirth, Anal. Chem.87, 2520–2526 (2015).
Mary J. Wirth is the W. Brooks Fortune Professor of Chemistry at Purdue University in West Lafayette, Indiana, USA. Alexis Huckabee and Jonathan Yasosky are graduate research assistants in the department.

New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)