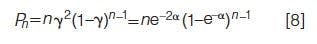Recent Developments in HPLC and UHPLC
Special Issues
Introduction from the Guest Editor.
When I was asked to edit a supplement for LCGC again, I was delighted. My background in physical chemistry has always made me captivated by separation theory; my career in industry has taught me to be practical in its use. My criterion to choose contributors is the same as always: I ask fellow researchers who conduct work that I find interesting. The selections here are quite varied, but they illustrate both the theory and the practical perspectives of separation science.
Mark Schure and Joe Davis explain statistical overlap theory to a point that it can be used in everyday chromatographic applications. This theory shakes the foundation for many practicing chromatographers in that it statistically evaluates the probability of a peak being pure. The results are not terrific. Simply, the authors show that for a moderately difficult separation the probability that a component of interest is resolved as a singlet peak on a single column is only 14%. All is not lost however; multiple columns can be used to increase this probability, although not as dramatically as I personally would like - the authors report the probability only increases to 52% for five columns in series. For those of us who love the theory of chromatography, this is a fascinating article. For the rest who know how important theory is to the practice of separations, this is an awakening and a reminder of what could be going on inside our chromatographic systems. Chromatographers take heed!
Every morning when we reach inside our bathroom cabinets, we are probably not awake enough to think about the chemistry behind the products we use. Brettell, Myers, and Pritchett, researchers at Cedar Crest College, have investigated liquid chromatography–mass spectrometry (LC–MS) for a series of parabens, BHT, and BHA. These compounds are used as preservatives in everyday products such as toothpaste, hand lotion, deodorant, foundation, hand sanitizer, and lipstick. Methyl and ethyl parabens were the most common preservatives and were found at the highest levels in deodorant and foundation samples. We should feel safer knowing that there is a way to make sure preservatives can be accurately measured in the personal care products we use daily.
Supercritical fluids were first used in chromatography in 1962 by Ernst Klesper, and enhancedâfluidity chromatography (EFC) was first examined by Susan Olesik and her group in 1991. Supercritical fluid chromatography (SFC) is when the mobile phase, either a pure or modified gas, is operated above its critical point. Enhanced-fluidity solvents are solvents that have added dissolved gases. Ironically, much of the literature using SFC with mixed solvents is mislabelled, as the temperature and pressures are too low for criticality. In actuality, these supercritical separations are conducted with subcritical conditions. For the practicing chromatographer, Olesik explains the theory behind the appropriate choice of solvent and operating conditions given the variety of options open to us: SFC, EFC, high performance liquid chromatography (HPLC), and even subcritical chromatography conditions.
At the frontier of column technology, there has always been a quest to control pH at extreme levels and adjust selectivity while maintaining long column lifetimes. Long, Mack, Wang, and Barber showed that by keeping a gradient constant and altering pH, the elution order of a group of eight acid, base, and neutral compounds could be dramatically changed and resolution improved with a superficially porous column. Positive ion electrospray mass spectrometry of basic compounds using high and low pH gradient HPLC showed improved peak shape and increased retention as well as signal and sensitivity increases. With these new superficially porous particle technology columns, separation scientists can examine a wider range of method development options. Here comes high efficiency, high speed, and durability, yeah!
With my coauthors Usher, Hansen, Bernstein, and Amoo, my laboratory has pursued the never-ending question of whether or not better precision is obtained when an internal standard is used instead of an external standard. In all of our experiments, the internal standard method significantly improved the precision. However, additional influencing factors on the precision are the injection volume and the method by which the internal standard is added to the analyte. Does this definitively settle this question? Only time will tell.
My hope is that you appreciate the articles in this supplement as much as I have taken pleasure in reading and editing them. These are some of my favourite scientists, who have challenged and continue to challenge my own experimental design as well as interpretation of results. I am confident that their advancements to the field of separation science will challenge you in your laboratory. Thank you to the authors - excellent work!

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)