The Recent Advances in Comprehensive Chromatographic Analysis of Emerging Drugs
LCGC North America
To address the challenges of analyzing new illicit drugs, emerging techniques such as UHPSFC with MS and UV detection, and GC with VUV detection, may be needed, particularly for distinguishing positional isomers and diastereomers.
The analysis of seized drugs containing emerging drugs, which are synthesized to skirt the controlled substances laws, is complicated by a large array of similar drugs including analogues, homologues, positional isomers, and diastereomers. Chromatographic techniques such as gas chromatography (GC) and liquid chromatography (LC), which are commonly used with mass spectrometry (MS) detection, have certain limitations-especially for positional isomers and diastereomers. This article discusses the use of emerging technologies that are complementary to established techniques, to significantly reduce these shortcomings for both synthetic cannabinoids and synthetic cathinones. In this vein, the utility of recently reported approaches including ultrahigh performance supercritical fluid chromatography (UHPSFC)–photodiode array (PDA) ultraviolet (UV)–MS, and GC–vacuum UV is discussed. To increase the specificity of analysis, multiple chromatographic techniques are commonly used. For the analysis of emerging drugs, a combination of GC and UHPSFC is recommended. The utility of a previously unreported coupled-columns approach for UHPSFC to significantly enhance resolution of synthetic cathinones is presented.
Emerging drugs are synthetically produced modifications of controlled drugs designed to skirt laws banning their use as recreational drugs. These changes often result in structural analogues, structural homologues, as well as positional isomers and stereoisomers. The identification of existing and new emerging drugs is complicated by the similarity in structure, lack of reference materials, insufficient libraries, lack of molecular ions for certain solutes (when electron ionization [EI] mass spectrometry [MS] is used), difficulty in deconvoluting spectra for coeluted solutes, and the similarity in MS spectra for diastereomers and positional isomers. Therefore, as a complement to MS detection, chromatographic resolution is particularly important. In this vein, gas chromatography (GC) is commonly used for the analysis of seized drugs, followed by liquid chromatography (LC) (specifically, high performance liquid chromatography [HPLC] and ultrahigh-pressure liquid chromatography [UHPLC]). Although GC has inherently higher resolving power (peak capacity) than HPLC, the former technique is disadvantageous for solutes (including many emerging drugs) that are thermally labile, polar, and nonvolatile.
For certain emerging drugs such as synthetic cathinones, GC–EI-MS suffers from extensive fragmentation in the source, which results in a lack of molecular ions, and a lack of diagnostic fragment ions (1). Differentiating between positional isomers for these solutes is particularly difficult using EI spectra, especially when substitution occurs on the benzene ring (2,3). Although LC–electrospray ionization (ESI)–MS/MS and LC–ESI-quadrupole time-of-flight (QTOF)-MS will provide molecular ions for synthetic cathinones, there is a scarcity of diagnostic fragment ions and difficulty in distinguishing between positional isomers (1). For other emerging drugs, such as synthetic cannabinoids, difficulty arises in distinguishing positional isomers using UHPLC–ESI-TOF-MS (4). Both GC–EI-MS (5) and LC–MS/MS (6) cannot distinguish between diastereomers. GC (4,7–9), HPLC (10,11), and UHPLC (4,12–14) have been used for the separation of emerging drugs. For mixtures of both controlled synthetic cannabinoids and controlled synthetic cathinones, neither GC nor reversed-phase UHPLC will resolve all these solutes, with GC slightly outperforming UHPLC (4,14). The controlled synthetic cathinones were poorly resolved using UHPLC in the hydrophilic interaction chromatographic (HILIC) mode (14). Both GC and reversed-phase UHPLC exhibit poor resolution for positional isomers of synthetic cannabinoids and synthetic cathinones (4,14), with better (but still incomplete) resolution of positional isomers of synthetic cathinones obtained using HILIC (14). GC (10) and HPLC (11) can exhibit poor resolution for diastereomers of synthetic cannabinoids.
Ultrahigh performance supercritical fluid chromatography (UHPSFC) uses SFC with packed columns that contain sub-3-µm particles, or columns that give equivalent performance such as core–shell, to produce highly efficient and rapid separations (15–18). Similar to LC techniques, SFC can analyze compounds that are thermally labile, polar, or nonvolatile without pretreatment or derivatization. Compared to UHPLC mobile phases, those used in UHPSFC are more diffusive with lower viscosities, which can result in separations that are up to four times faster.
The utility of ultraviolet (UV) detection subsequent to HPLC and UHPLC separation to aid in the identification of emerging drugs, including positional isomers on the benzene ring, has been described (2,12,19). Vacuum UV (VUV) detection, which is easily interfaced to GC, can measure solute absorbance between 120 and 430 nm, and is capable (in contrast to conventional UV detection) of probing σ→σ*, and short wavelength π→π* transitions (20). VUV detection can not only demonstrate class similarities, but also allows for differentiation within a class, including most isomers (21). In addition, measured spectra can be matched against an existing library to identify the compounds (21). Furthermore, since UV spectra are additive, spectra can also be easily deconvoluted if coelution exists (22). The identification of coeluted compounds by GC–EI-MS would require altering chromatographic conditions, including changing columns, or using multidimensional GC, which may not be readily available (21,22).
Coupled columns (two or more columns, with or without the same stationary phase combined in series) can be used to improve separations by a linear increase in peak capacity. The same mobile phase can be used throughout both columns unlike in multidimensional LC where different mobile phases are usually required (23,24). However, for multidimensional chromatography, unlike for coupled columns, peak capacity is the product of the peak capacity of the individual columns. Because SFC uses mobile phases of significantly lower viscosity, there is less concern for back-pressure drops, which facilitates the use of coupled columns (24).
In this article various approaches are discussed to provide improved chromatographic resolution for emerging drugs, particularly for positional isomers and diastereomers, including the use of UHPSFC both in the single and tandem column mode. In addition, for synthetic cathinones, the use of UV detection for UHPSFC in tandem with MS detection, and VUV detection for GC to complement MS detection, which is particularly useful for positional isomers, is discussed.
The Utility of UHPSFC for the Analysis of Emerging Drugs
UHPSFC has been used for the separation of synthetic cannabinioids (25,26) and synthetic cathinones (14,27). For a large array of these solutes, including sets containing up to 10 positional isomers and difficult-to-separate diastereomeric compounds, UHPSFC has been compared to both GC and UHPLC (4,10,11,14,26). For these latter studies, various columns and mobile phase conditions were evaluated. The columns included four achiral stationary phases including Acquity UPC2 Torus 2-PIC, Acquity UPC2 Torus Diol, Acquity UPC2 Torus DEA, and Acquity UPC2 Torus 1-AA (100 mm × 3.0 mm, 1.7 µm), and three chiral columns, including Acquity UPC2 Trefoil AMY1, Acquity UPC2 Trefoil CEL1, and Acquity UPC2 Trefoil CEL2 (150 mm × 3.0 mm, 2.5 µm) (all from Waters). For the mostly neutral synthetic cannabinoids (JSW-200 basic) a 5-min gradient was conducted (usually with a 1-min hold) using carbon dioxide with methanol, ethanol, isopropanol, or acetonitrile as a modifier. A fine-tuned separation was obtained by varying gradient steepness (time of gradient), and temperature. For the basic synthetic cathinones, 5-min gradients (1-min hold) were conducted or isocratic analysis (usually under 6 min) using carbon dioxide with methanol, ethanol, or isopropanol as the modifier or ammonium formate or ammonium hydroxide as the additive. A fined-tuned separation was obtained by varying the temperature. For the gradient separations, conditions were adjusted so that the chromatographic peaks occupied approximately the entire gradient time.
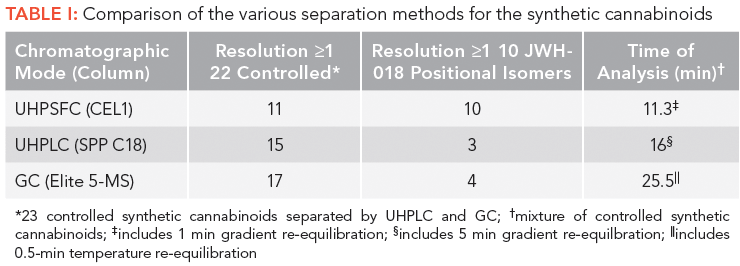
It is of interest to compare the separation of the synthetic cannabinoids by UHPSFC, GC, and UHPLC (see Table I). Of particular interest for the synthetic cannabinoids were the separations of JWH-018 and nine positional isomers, and the diastereomers such as HU210 and HU211. For this purpose, the polysaccharide-based chiral columns performed well, allegedly because of their selectivity arising from dipole–dipole and steric interactions (26). The near- baseline separation of JWH-018 and nine positional isomers, as well as the well-resolved separation of HU-210 and HU-211 are shown in Figures 1a and 1b, respectively. In comparison for JWH-018 and it positional isomers, GC (5% phenyl column) resolved four out of 10 solutes, while at best UHPLC (reversed-phase mode with a C18 column) resolved three out of 10 (4). HU-210 and HU-211 were not resolved using GC (10) or HPLC (11). In regards to the separation of a diverse mixture of controlled synthetic cannabinoids, 11 out of 22 were separated (resolution ≥ 1) using UHPSFC (10-min gradient plus 1-min gradient reequilibration) using the same system employed for JWH-018 and it positional isomers (26). In contrast, 17 out of 23 were separated (resolution ≥ 1) using GC for the same system used for the positional isomers (24-min temperature-programmed run plus 0.5-min temperature reequilbration), while at best 15 out of 23 were separated (resolution ≥ 1) using UHPLC (11-min gradient plus 5-min gradient equilibration) (4).
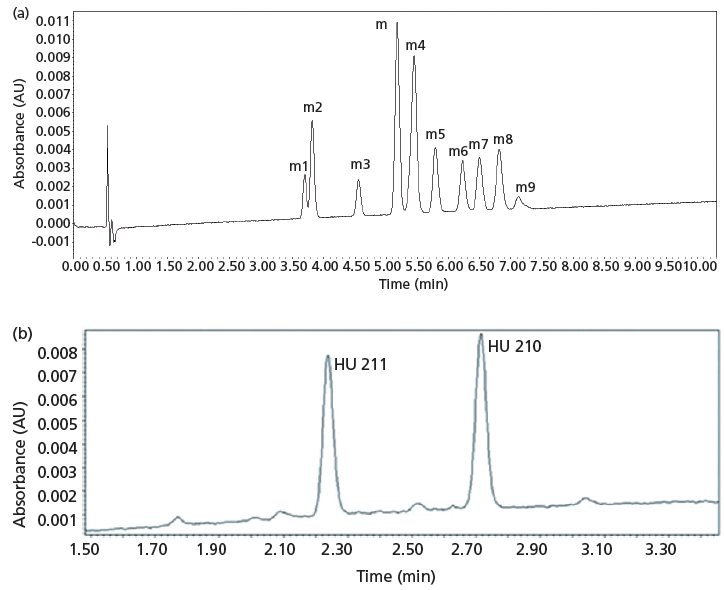
Figure 1: (a) UHPSFC separation of JWH 018 and nine of its positional isomers. Column: 15 cm × 3.0 mm, 2.5-µm d p Acquity UPC2 Trefoil CEL1; initial mobile-phase conditions: 20% isopropanol, 80% carbon dioxide; final mobile-phase conditions: 31% isopropanol, 69% carbon dioxide; gradient: 10.3 min, linear, with a 1.0-min gradient reequilibration; flow rate: 1.25 mL/min; temperature: 55 °C; automated back-pressure regulator setting: 2200 psi; detection: UV absorbance at 273 nm; injection volume: 1 µL. Peaks (5 µg/mL ): m = JWH-018, m1 = JWH 018 2'-naphthyl-N -(1, 2-dimethylpropyl) isomer, m2 = JWH 018 2'-naphthyl-N -(1 ethylpropyl) isomer, m3 = JWH 018 2'-naphthyl-N -(1 methylbutyl) isomer, m4 = JWH-016, (m5) JWH 018 2'-naphthyl-N -(1,1-dimethylpropyl) isomer, m5 = JWH 018 2'-naphthyl-N -(1,1-dimethylpropyl) isomer, m6 = JWH 018 2'-naphthyl-N -(2 methylbutyl) isomer, m7 = JWH 018 2'-naphthyl-N -(2, 2-dimethylpropyl) isomer, m8 = JWH 018 2'-naphthyl-N -(3 methylbutyl) isomer, m9 = JWH 018 2'-naphthyl isomer. (b) UHPSFC separation of HU-210 and HU-211. Column 15 cm × 3.0 mm, 2.5-µm d p Acquity UPC2 Trefoil AMY1; initial mobile-phase conditions: 18% isopropanol, 80% carbon dioxide; final mobile-phase conditions: 53% isopropanol, 82% carbon dioxide; gradient: 5 min, linear, hold for 1 min, 1.0 min gradient reequilibration; flow rate: 1.25 mL/min; temperature: 45 °C; automated back-pressure regulator setting: 2200 psi; detection: UV absorbance at 273 nm; injection volume: 1 µL. Adapted with permission from reference 26.
Similarly, it is of interest to compare the separation of the synthetic cathinones by UHPSFC, GC, and UHPLC (see Table II). To evaluate the utility of UHPSFC for the separation of positional isomers of synthetic cathinones, nine mixtures containing different sets of masses for a total of 34 positional isomers were investigated (14). The criterion used to evaluate a chromatographic system was to sum the total number of isomers resolved with a resolution ≥ 1 for each mixture. Using a diol column operating under isocratic conditions, with the mobile phase containing a methanol modifier and an ammonium formate additive, 28 out of 34 positional isomers were separated. Since the synthetic cathinones are chiral, an achiral column was preferred to reduce the complexity of the separation. In practice, since all optical isomers of a given synthetic cathinone are controlled, enantiomeric resolution is of limited forensic value. In comparison, GC with a 5% phenyl column resolved 22 out of 34 positional isomers, while at best UHPLC (HILIC mode with a HILIC column) resolved 27 out of 34 (14). Note that UHPLC using the commonly employed reversed-phase mode with a C18 column only resolved 7 out of 34. For the separation of a diverse mixture of controlled synthetic cathinones, 11 of 15 were separated (resolution = 1) using UHPSFC (8-min isocratic run using the same system employed for the positional isomers) (14). For the same systems used for the positional isomers, 13 out of 15 were separated by GC (13-min temperature program plus a 0.5-min temperature reequilbration), while 12 out of 15 and five out of 15 were obtained using UHPLC in the reversed-phase mode (11-min gradient plus a 5-min gradient reequilibration) and HILIC modes (12-min isocratic), respectively (14).
For the analysis of seized drugs, it is important to use more than one non-correlated chromatographic system to decrease the likelihood of the misidentification of a solute of interest. In this vein, the orthogonality of UHPSFC to both GC and UHPLC for both synthetic cannabinoids and synthetic cathinones was examined. For this purpose, principal component score plots were generated for mixtures of the controlled substances of both the synthetic cannabinoids (26) and synthetic cathinones (14). For both classes of drugs, the UHPSFC systems stand by themselves, highly orthogonal to both GC and UHPLC using a C18 column operating in the reversed-phase mode. For synthetic cathinones, UHPSFC is also highly orthogonal to UHPLC using a HILIC column operating in the HILIC mode. Given that UHPLC in the reversed-phase mode could provide poor overall resolution of positional isomers and that UHPLC in the HILIC mode could provide poor resolution of a diverse mixture of controlled substances, a viable combination of chromatographic techniques for the analysis of emerging drugs would be GC and UHPSFC.
The use of serial coupled columns with different stationary phases (Diol + 2PIC) for UHPSFC significantly improved the overall resolution of synthetic cathinones. Using a previously reported experimental setup for single columns (14), a mixture of 15 controlled synthetic cathinones was resolved (Figure 2). In addition, under the same experimental isocratic conditions, 32 out of 34 positional isomers were also resolved.
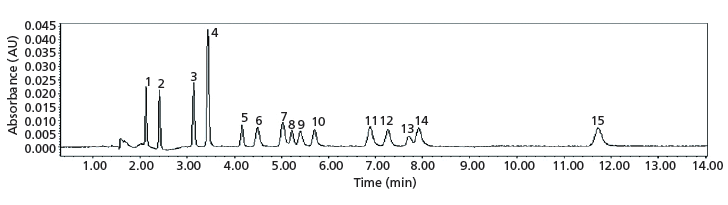
Figure 2: Coupled-column chromatogram of a standard mixture of synthetic cathinone controlled substances. Columns: 10 cm × 3.0 mm, 1.7-µm d p Acquity UPC2 Torus Diol connected in series with a 10 cm × 3.0 mm, 1.7-µm d p Acquity UPC2 Torus 2-PIC column; mobile phase: 3% methanol (10 mM ammonium formate in methanol), 97% carbon dioxide; flow rate: 1.00 mL/min; temperature: 40 °C; automated back-pressure regulator setting: 1800 psi; detection: UV absorbance at 230 nm; injection volume: 0.5 µL. Peaks (50 µg/mL concentration): 1 = α-PVP, 2 = α-PBP, 3 = MDPV, 4 = naphyrone, 5 = 4-MePPP, 6 = pentedrone, 7 = buphedrone, 8 = 3-fluoromethcathinone, 9 = 4-methylethcathinone, 10 = 4-fluoromethcathinone, 11 = pentylone, 12 = methcathinone, 13 = butylone, 14 = mephedrone, 15 = methylone .
The Use of Complementary Detection Schemes for Both UHPSFC and GC Analysis of Synthetic Cathinones
Not only are GC and UHPSFC highly complementary separation techniques for emerging drugs such as synthetic cathinones, but the choice of detection techniques can also provide essential complementary information. Along these lines, for UHPSFC the use of photodiode-array (PDA) UV detection in tandem with ESI-MS detection provides complementary data to GC with EI-MS detection. In contrast to GC–EI-MS detection for synthetic cathinones, UHPSFC with tandem UV and ESI-MS detection will provide molecular mass information as well as distinguish between positional isomers where substitution occurs on the benzene ring. The great utility of UV detection for positional isomers (for example, molecular mass 191) is shown in Figure 3. For compounds that differ in substitution on the benzene ring, such as 2-ethylmethcathinone, 2,4-dimethylmethcathinone, 2,3-dimethylmethcathinone, and 3,4-dimethylmethcathinone, unique UV spectra are obtained. It should be noted that these solutes are resolved by both UHPSFC and GC, further adding to the specificity of their determination (14). 4-methylethcathinone and 4-methylbuphedrone, which differ in substitution on the aliphatic portion of the molecule, give identical UV spectra. UV spectra can also help identify which class or subclass of an emerging drug is present (3,22), which is an especially useful aid for the identification of unknown compounds.
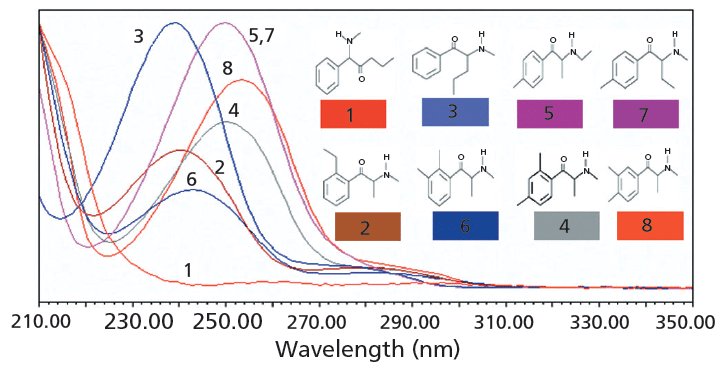
Figure 3: Normalized UV spectra of synthetic cathinone positional isomers with molecular mass 191 acquired by PDA-UV detection subsequent to UHPSFC separation. Column: 10 cm × 3.0 mm, 1.7-µm dp Acquity UPC2 Torus Diol; mobile phase: 3% methanol (10 mM ammonium formate in methanol), 97% carbon dioxide; flow rate: 1.25 mL/min; temperature: 40 °C; automated back-pressure regulator setting: 2200 psi; injection volume: 0.5 µL. Spectra: 1 = isopentedrone, 2 = 2-ethylmethcathinone, 3 = pentedrone, 4 = 2,4-dimethylmethcathinone, 5 = 4-methylethcathinone, 6 = 2,3-dimethylmethcathinone, 7 = 4-methylbuphedrone, 8 = 3,4-dimethylmethcathinone. Adapted with permission from reference 3 and John Wiley and Sons.
With the mobile-phase conditions used with UHPSFC to acquire UV spectra, there was at least a 10-nm blue shift (shift to shorter wavelengths) compared to UHPLC mobile-phase conditions (3). This effect was largely caused by the presence of carbon dioxide as a predominant component in the mobile phase, as demonstrated by the much smaller blue shift observed when carbon dioxide was replaced with methanol under otherwise identical mobile-phase conditions. The trends in the data can be explained as the result of the dielectric constants of the media surrounding the analyte molecules. Methanol is a highly polar solvent with a high dielectric constant of 32.6 at 20 °C (28). In contrast, supercritical carbon dioxide with a small dipole moment has a small dielectric constant of approximately 1.45 at 40 °C (29). Kadum and colleagues (30), who explored the effects of nonpolar and polar solvent on the π→π* transitions of benzaldehyde and acetophenone (solutes with a similar chromaphore to the synthetic cathinones), reported that the π→π* transitions shifted to longer wavelengths with increasing solvent dielectric constant.
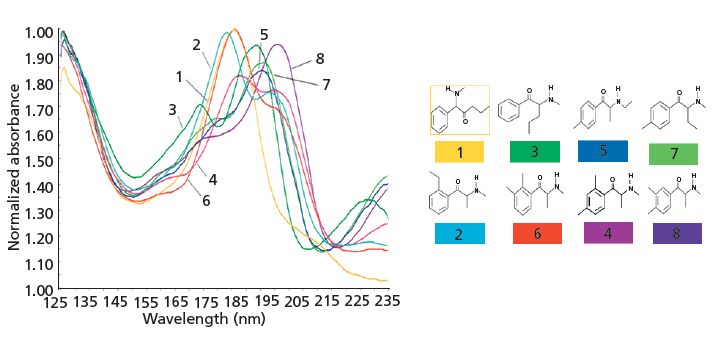
Figure 4: Normalized VUV spectra of synthetic cathinone positional isomers with molecular mass 191 acquired by VUV detection subsequent to GC separation. Column: 30 m × 0.25 mm, 0.25-µm df PerkinElmer Elite-5MS; inlet temperature: 230 °C; injection volume: 2 µL (1:10 split); oven program: 80 °C initial temperature for 1.0 min, ramp to 320 °C at a rate of 20 °C/min, and a temperature hold for 1.5 min; VUV system temperature :300 °C; VUV makeup gas pressure: 0.5 psi. Adapted with permission from reference 31.
In contrast to conventional UV detection for supercritical fluid or liquid-phase separation techniques, VUV detection examines enhanced π→π* as well as σ→σ* transitions for gas-phase separations. Therefore, the latter technique is not only capable of distinguishing between positional isomers where substitution occurs on the benzene ring, but also of distinguishing between positional isomers of synthetic cathinones where substitution also occurs on the aliphatic portion of the molecule (31). As shown in Figure 4, all of the eight m/z 191 positional isomers, in contrast to UV detection, yield unique VUV spectra, including 4-methylethcathinone and 4-methylbuphedrone, which differ in substitution on the aliphatic portion of the molecule. It should be noted (see Figure 5) that two of the solutes, 4-methylbuphedrone and 2,3-dimethylmethcathinone, are coeluted in the GC separation. A nice feature of VUV detection is the ability to deconvolute coeluted peaks and obtain the subsequent individual VUV spectra (32). Therefore, another viable approach for the analysis of emerging drugs would be to use UHPSFC with MS/MS or QTOF detection and GC–VUV detection. This complementary detection scheme would provide molecular mass information, diagnostic fragmentation, information about the class or subclass of emerging drugs, and the ability to distinguish between positional isomers. In addition, similar to UV detection, VUV detection is excellent for quantitative analysis (31). Unlike flame ionization detection for GC, which is also excellent for quantitative analysis, VUV detection provides qualitative information besides retention time, including possible coelution (31).
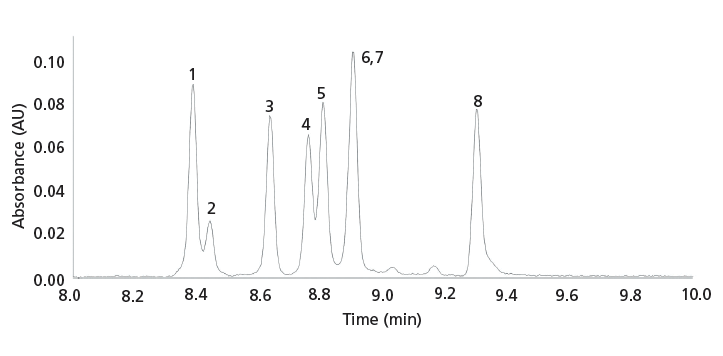
Figure 5: GC-VUV separation of synthetic cathinone positional isomers with molecular mass 191. See Figure 4 for GC conditions and peak identity. Adapted with permission from reference 31.
Conclusion
The ability to distinguish between emerging drugs using chromatographic techniques with various detection schemes has been recently enhanced by emerging techniques such as UHPSFC and VUV detection. These approaches are particularly useful for distinguishing between positional isomers. For the identification of regioisomers, the coupling of GC with VUV and MS would be useful.
Funding
The work reported was supported in part by Award No. 2014-R2-CX-K009, awarded by the National Institute of Justice, Office of Justice Programs, and the U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this article are those of the authors and do not necessarily reflect those of the Department of Justice. The work was also supported in part by PerkinElmer Corporation through GWU Proposal #13-04142.
Acknowledgments
The authors would like to thank Waters for the loan of the UHPSFC instrumentation. They would also like to thank PerkinElmer Corporation for donating the UHPLC columns.
References
(1) D. Zuba, TrAC, Trends Anal. Chem. 32, 15–30 (2012).
(2) E. Kohyama, T. Chikumoto, H. Tada, K. Kitaichi, T. Horiuchi, and T. Ito, Anal. Sci. 32, 831–837 (2016).
(3) W.F. Rowe, I. Marginean, S. Carnes, and I.S. Lurie, Drug Test.Anal. 9, 1512–1521 (2017).
(4) I. Marginean, I.W, Rowe, and I.S. Lurie, Forensic Sci. Int. 249, 83–91 (2015).
(5) https://www.caymanchem.com/Home
(6) P. Lebel, K. Waldron, and A. Furtos, Current Trends in Mass Spectrometry supplement to LCGC North Am., LCGC Europe, and Spectroscopy 13(1), 8–14 (2015).
(7) K. Tsujikawa, T. Miluma, K. Kuwayama, H. Miyaguchi, T. Kanamori, Y. Iwata, and H. Inoue, Drug Test. Anal. 5, 670–677 (2013).
(8) N.N. Daeid, K.A. Savage, D.C. Ramsay, C. Hollabd, and O.B. Sutcliff, Sci. Justice 54, 22–31 (2014).
(9) S.Kerrigan, M. Savage, C. Cavazos, and P. Bella, J. Anal. Toxicol . 40, 1–11 (2016).
(10) B.K. Logan, L.E. Reinhold, A. Xu, and F.X. Diamond, J. Forensic Sci. 57, 1168–1180 (2012).
(11) L.A. Ciolino, J. Forensic Sci. 60, 1171–1181 (2015).
(12) L. Li, and I.S. Lurie, Forensic Sci. Int. 237, 100–111 (2014).
(13) C. Clyde, S. Blake, S. O'Brien, I. Igwilo and I.S. Lurie, Anal. Methods 7, 9763–9772 (2015).
(14) S. Carnes, S. O'Brien, A. Szewczak, L. Tremeau-Cayel, W.F. Rowe, B. McCord and I.S. Lurie, J. Sep. Sci. 40, 3545–3556 (2017).
(15) A.G.G. Perrenoud, J. Boccard, J.L. Veuthey, and D. Guillarme, J. Chromatogr. A 1262, 205–213 (2012).
(16) A.G.G. Perrenoud, J.L.Veuthey, and D. Guillarme, J. Chromatogr. A 1266, 158–167 (2012).
(17) C. Gourmel, A.G.G. Perrenoud, L. Waller, E. Reginato, J. Verne, B. Dulery, J.L. Veuthey, S. Rudaz, J. Schappler, and D. Guillarme, J. Chromatogr. A 1282, 172–177 (2013).
(18) A.G.G. Perrenoud, C. Hamman, M. Goel, J.L. Veuthey, D. Guillarme, and S. Fekete, J. Chromatogr. A 1314, 288–297 (2013).
(19) S.D. Brandt, S.P. Elliot, P.V. Kavanagh, N.M. Dempster, M.R. Meyer, H.M. Maurer, and D.E. Nichols, Rapid Commun. Mass Spectrom. 29, 573–584 (2015).
(20) K.A. Schug, I. Sawicki, D.D. Carlton, H. Fan, H.M. McNair, J.P. Nimmo, P. Kroll, J. Smuts, P. Walsh, and D. Harrison, Anal. Chem . 86, 8329-8335 (2014).
(21) B. Gruber, T. Groeger, D. Harrison, and R. Zimmermann, J. Chromatogr. A 1464, 141–146 (2016).
(22) J. Schenk, J.X Mao, J Smuts, P. Walsh, P. Kroll, and K.A. Schug, Anal. Chim. Acta 945, 1–8 (2016).
(23) T. Alvarez-Segura, J.R. Torres-Lapasio, C. Ortiz-Bolsico, M.C. Garcia-Alvarez-Coque, Anal. Chim. Acta 923, 1–23 (2016).
(24) K.W. Phinney, L.C. Sander, and S.A. Wise, Anal. Chem. 70, 2331–2335 (1998).
(25) T. Toyooka and R. Kikura-Hanajiri, Chem. Pharm. Bull. 63, 762–769 (2015).
(26) S. Breitenbach, W.F. Rowe, B. McCord, and I.S. Lurie, J. Chromatogr. A 1440, 201–211 (2016).
(27) V. Pauk, V. Zihlova, L. Borovcova, V. Havlicek, K. Schug, and K. Lemr, J. Chromatogr. A 1423, 169–176 (2015).
(28) I.M. Smallwood, Handbook of Organic Solvent Properties (Halsted Press, New York, New York, 1996).
(29) Y. Zhang, J. Yang, and Y. Yu, J. Phys. Chem. B 109, 13375–13382 (2005).
(30) S.A. Kadum, N.A. Baker, and T.E. Al-Edanee, Monatshefte fur Chemie 122, 349–358 (1991).
(31) L. Skultety, P. Frycak, C. Qiu, J. Smuts, L. Shear-Laude, K. Lemr, J.X. Mao, P. Kroll, K.A. Schug, A. Szewczak, C. Vaught, I. Lurie, and V. Havilicek, Anal. Chim. Acta 971, 55–57 (2017).
Ira S. Lurie and Walter F. Rowe are with the Department of Forensic Sciences at George Washington University in Washington, D.C. Lauriane Tremeau-Cayel is an Associate Scientist 11 with Cayman Chemical in Ann Arbor, Michigan. Direct correspondence about this article to: islurie@email.gwu.edu

Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.











