The Rebirth of Chromatography 75 Years Ago
LCGC North America
After a 25-year dormant period, chromatography was finally reborn in the first years of the 1930s.
Mikhail Tswett developed chromatography in the first years of the 20th century. His first report was a lecture in March 1903 in Warsaw, before an audience of 41 colleagues and students, followed three years later by his seminal twin publications (1). For some time, however, botanists and chemists were skeptical about his results. In the first 25 years, only a few scientists tried to utilize the technique in their laboratories, while others did their best to discredit its usefulness. The situation was best illustrated by the remark of F.M. Schertz, an American agricultural chemist, who stated as late as in 1929 that "it is evident that Tswett was never at any time dealing with pure pigments, for not once were the substances crystallized" (2).

Leslie S. Ettre
Just a couple of years after this negative comment chromatography suddenly was found to be suited ideally for the study of complex natural substances and the separation of the individual pigments: this rebirth of chromatography started in Heidelberg, Germany, and was initiated by the activities of Edgar Lederer in the laboratories of Richard Kuhn. Their work was closely followed by Paul Karrer, in Zurich, Switzerland, László Zechmeister, in Pécs, Hungary, and by a real explosion in the use of chromatography. Within less than a decade it became a generally accepted technique in both Europe and the United States. This change in the attitude is best illustrated by a statement of Paul Karrer, the great Swiss chemist, who in 1939 — just 10 years after Schertz' condemnation — clearly stated that (3):
It would be undoubtedly a mistake to believe that a preparation purified by crystallization should be purer than one prepared through chromatographic analysis. All recent investigations have shown that chromatographic purification is much more superior over purification by crystallization.
The present report deals with this golden decade, with the activities of Kuhn, Karrer and Zechmeister and their associates, who 75 years ago laid down the foundation upon which all the future developments of chromatography were based. We start with the activities of Richard Kuhn's group in Heidelberg, in 1930–1931.

Richard Kuhn
A native of Vienna, Austria, Richard Kuhn (1900–1967) earned the Ph.D. degree from the University of Munich, Germany, under Richard Willstätter (1872–1942), the most famous German chemist of that time. After a few years of continuous association with this university, Kuhn was offered in 1926 upon the recommendation of Willstätter full professorship at the Swiss Federal Technical University (E.T.H.), in Zurich. Then three years later Kuhn moved to Heidelberg, Germany, where he was appointed as the director of the Chemistry Institute within the Kaiser Wilhelm Institute for Medical Research. (This institute actually consisted of four sub-institutes, the Chemistry Institute being one of these.) From Zurich he took with him several assistants, among them most notably Alfred Winterstein (1889–1960), and within a short time he further expanded the scientific staff of his institute.
While still in Zurich, Kuhn's interest turned toward the complex plant pigments called carotenoids, compounds with long chains of carbon atoms with alternating single and double bonds. He continued this work in Heidelberg. The rebirth of chromatography is associated with these activities.
The Field of Carotenoids
Although their existence has been known for a long time, very little was known about these compounds. In 1907, Willstätter and Mieg (4) clearly established the existence of two such compounds: carotene, a hydrocarbon with the elementary composition of C40H56, and xanthophyll, with the composition of C40H56O2. Then in 1912, Willstätter and Escher (5) found another compound with the same composition as carotene, which they called lycopene . Tswett also studied these compounds, but contrary to Willstätter, in his 1910 book he expressed the opinion that carotene is not a single compound but a mixture of two or more homologues which could be separated by chromatography (6). Then in a paper published in 1911 Tswett stated that xanthophyll is not a pure compound either, but a mixture of some isomeric compounds (7). It is interesting to note that Willstätter disputed this opinion, saying that "it is unlikely that this assumption of the respected botanist (that is, Tswett) is true" (8). However, as we shall see, he was wrong. It was also Tswett who, in 1911, proposed to use the term carotenoids to express this group of compounds (7).
This confusion about the various carotenoid compounds existed until the early 1930s: such compounds were observed in various plants, but it was not clear whether compound A found in source X is identical or not to compound B found in source Y, and whether a recently isolated substance is a pure compound or a mixture of some isomeric compounds. This is clear from the monograph of L.S. Palmer published in 1922, representing the first relatively modern summary of the information available about carotenoids (9): this book reported on the compounds found in various plants and animals, but the information presented was mainly empirical. It is important to realize that none of these researchers had any information about the actual molecular structures of these compounds; these only were established gradually in the 1930s by Kuhn, Karrer, and others (Figure 1).

Figure 1
A special problem in the investigation of carotenoids was that these compounds are present in nature only in very small amounts. Therefore, when using classical methods in their study, very large amounts of the original substance had to be processed. For example, in 1912, Willstätter and Escher had to extract 6000 hen eggs to obtain a crude, crystalline pigment for further investigation (5). It was Palmer's particular merit that in his book he pointed out the usefulness of chromatography in the separation of these compounds, and was giving a detailed description of the technique; however, until the work in Heidelberg, in 1930, practically nobody followed Palmer's advice.
We also should mention that some of the interest in the carotenoids stemmed from nutrition studies (this is how Palmer became involved) and from observations connecting the carotenoids to vitamin A activity. These investigations further intensified after Hans von Euler-Chelpin at Stockholm University, in 1928 (10), and Thomas Moore, at Dunn Nutrition Laboratory, in Cambridge, England, in 1930 (11), clearly stated that vitamin A is produced in nature from carotene.
Edgar Lederer and the Rebirth of Chromatography
Also a native of Vienna, Edgar Lederer (1908–1988) studied chemistry at the University of Vienna, receiving the Ph.D. degree in 1930. He then accepted a postdoctoral position in Kuhn's institute. At that time their newest research showed similarities of the pigments present in egg yolk and the yellow xanthophylls of green leaves studied some 25 years earlier by Willstätter. The situation was further complicated by a recent paper of Paul Karrer (12), at the University of Zurich, describing a new "xanthophyll" isolated from yellow corn which he named zea-xanthin. Lederer's first job was to carry out laboratory investigations to clear up this question. The subsequent events are well documented in two recollections of Lederer (13,14).
Lederer started his investigations by measuring the absorption spectra, melting points and optical rotations of various carotene preparations from carrots, lutein from egg yolk, xanthophylls from green leaves, and zeaxanthin from yellow corn; he found that the last two pigments had distinctly different properties, while lutein seemed to lie between the two. Discussing the results, Kuhn had a suggestion: perhaps the lutein of egg yolk might actually be a mixture of leaf xanthophyll and corn zeaxanthin. Naturally, this could be proven by the separation of the "lutein" of egg yolk into the two components. But how?
Lederer was not familiar with the field of carotenoids (his thesis work in Vienna dealt with indole alkaloids) and therefore, he first had to study the existing literature. In Palmer's carotenoid book (9), he found favorable information about the chromatographic adsorption technique of Tswett. He also read the chlorophyll book of Willstätter and Stoll (8) expressing their misgivings about the technique and Tswett's results, and the publications of Dhéré, Palmer, and Coward reporting on its successful use. Somewhat confused by these contradictory reports he went to Kuhn, asking his advice. At that time Kuhn remembered the manuscript copy of the German translation of Tswett's 1910 book (published in Russian) (6) specially made for Willstätter soon after its publication, what his teacher gave him many years ago. Fortunately he could still find it and he gave the copy to Lederer for study. After reading it, Lederer decided to try to apply the chromatographic technique to solve his problem.
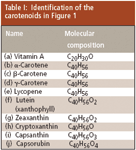
Table I: Identification of the carotenoids in Figure 1
In his recollections, Lederer (14) vividly describes the event on a day in December 1930, when he prepared a column packed with CaCO3 according to Tswett's instructions, then poured the carbon disulfide solution of a mixture of 0.5 mg of pure lutein and 0.5 mg of zeaxanthin onto this column, and washed it with the same solvent: soon a large orange band became visible, with distinctly different hues in the upper and the lower part. He carefully scraped off the two layers, dissolved the pigments in methanol, and "lo-and-behold, the upper zone had the spectrum of lutein, and the lower the spectrum of zeaxanthin." As the next step he prepared crude lutein extract of the egg yolk of 100 eggs (the white of which was previously "transformed into a delicious cake") and chromatographed it on a column of 7-cm diameter. (Note that he needed only 100 eggs as compared to 6000 eggs used in 1912 by Willstätter and Escher when using classical chemical methods!) After breaking the packing into zones, extracting the pigments from them and separately rechromatographing the individual extracts, pure lutein and zeaxanthin were obtained. These experiments proved that egg yolk indeed contained the two carotenoids, and that Tswett's chromatographic method can be used for preparative separation. In this way, he also confuted Willstätter who, just a few years earlier, specifically stated that "we can consider the chromatographic adsorption analysis ... not suitable for work in larger scale, that means for preparative purposes" (15).
Parallel to this work Lederer also used chromatography on a column now packed with alumina, for the separation of α- and β-carotenes. (It already had been shown by Tswett that carotene will go through CaCO3 without any retardation and therefore, another adsorbent is needed.)
These chromatographic results were summarized in three fundamental papers. The first was just one-page long, indicating the possibility of the separation of the two carotene isomers; it was submitted to Naturwissenschaften on February 17, 1931, and it mentions the use of chromatography only in a single sentence (16). This was then followed by two papers submitted on March 10 and 18, respectively, describing in detail the experiments about the separation of xanthophylls (17) and isomeric carotenes (18). These three papers represent the rebirth of chromatography.
Kuhn also proposed a change in the nomenclature for the carotenoids: to use the name xanthophyll as a generic name for all oxygenated C40 carotenoids and retain the name lutein only for the major constituent of leaf xanthophylls (17). Eventually this nomenclature was adopted by the International Union of Pure and Applied Chemistry (IUPAC).

Figure 2
Further Activities of the Heidelberg Group
Unfortunately, the happy and productive group of Kuhn in Heidelberg (Figure 2) did not last for long: after Adolf Hitler came to power in Germany, in 1933, major changes were forced to occur. Within a few weeks Edgar Lederer had to flee because of his leftist political activities and Jewish origin: indeed, in a few days the Gestapo came to arrest him. Because his wife was French, they went to France where he worked for two years in various research laboratories. In 1935, he moved to the Soviet Union as the research director of the Vitamin Institute in Leningrad, but two years later he returned to France and became associated with the Centre National de la Recherche Scientifique (CNRS). Lederer survived the war and German occupation in the French countryside, escaping a number of times police and Gestapo raids. He returned to Paris after the war, advancing within the CNRS, and starting to teach at the Sorbonne. From 1960 on, he has been the director of the Institut de Chimie des Substances Naturelles of the CNRS. In 1963, Lederer received the Wilhelm von Hoffmann Gold Medal of the German Chemical Society and on that occasion he met again Richard Kuhn, his boss 30 years earlier (Figure 3), who, as president of the Society, personally presented him the medal with the following words (19): "In Heidelberg, in my institute, you separated α- and β-carotene, and resuscitated the method of chromatography."

Figure 3
Lederer remained active in Paris for the rest of his life. In September, 1988, he was scheduled to present the keynote lecture at the 17th International Chromatography Symposium, in Vienna, but could not travel because of illness. He died soon after.
In 1934 Winterstein had to leave Heidelberg and return to his native Switzerland where he joined Hoffmann–La Roche, at that time the largest pharmaceutical house in the world, as their chief chemist. In the 1930s, he often served as the rowing ambassador of chromatography: A.J.P. Martin remembers that while a student at Cambridge University, he attended a lecture given by Winterstein demonstrating the separation of crude carotene into its components (20).
Richard Kuhn remained in Heidelberg for his whole life, and in 1937 became director of the parent Kaiser Wilhelm Institute for Medical Research. He continued to contribute significantly to our knowledge of carotenoids, isolating a number of previously unknown compounds of this group and establishing their structures. In 1938, he was selected to obtain the Nobel Prize in Chemistry "for his results in the field of carotenoids and vitamins," but by then Hitler did not permit Germans to accept the prize and Kuhn could receive it only after the war. He remained active practically until his death in 1967.
Paul Karrer
When the Heidelberg group reported on the use of chromatography for carotenoid separation, Kuhn was already in a fierce, but gentlemanly scientific competition with Paul Karrer, professor at the University of Zurich, Switzerland, who also was involved in the study of complex plant pigments.
Paul Karrer (1889–1971) (Figure 4) was born in Moscow, Russia. His parents were Swiss nationals and his father was a dentist practicing in Russia, but in 1892 the Karrer family returned to Switzerland. Paul studied chemistry at the University of Zurich, receiving the Ph.D. degree in 1911. His doctorate thesis dealt with organic arsenic compounds, and this work drew the attention of Paul Ehrlich (1834–1915), the great German chemist — the father of what we today call chemotherapy. In 1909, Ehrlich developed Salvarsan, also an organic arsenic compounds, to cure syphilis. Ehrlich invited Karrer to his Institute of Experimental Therapy, in Frankfurt am Main, Germany, as his assistant. Upon Ehrlich's death in 1915, Karrer took over the position of the research director of the Institute, but in 1918, he returned to Zurich: he was appointed a full professor and the director of the university's Chemistry Institute, a position he held until his retirement, in 1959.

Figure 4
Besides being an exceptional researcher, Karrer also excelled as a teacher. His Lehrbuch der Organischen Chemie first published in 1927 has served in many countries as the standard textbook of organic chemistry for over two decades. It went through 13 editions and was translated into seven languages; I also used it as a student at the Technical University of Budapest, Hungary, in 1942–1944.
Karrer's early research after returning to Zurich dealt with peptides, but by the mid-1920s he started his investigations on vitamin A and carotenoids. By 1930–1931 he elucidated the structure of β-carotene and vitamin A and has shown that the latter is formed in the body from the carotene, by splitting its molecule into two (21,22).
Structure elucidation is based upon chemical investigations, but naturally, one needs a pure compound for it. Therefore it is understandable, that Karrer adopted chromatography for his work almost immediately after the first publication of Kuhn's group. In his two 1931 papers (21,22) dealing with the structure elucidation of vitamin A, Karrer already used chromatography on Al2O3 to purify the extract of fish liver oil containing the vitamin. He also pointed out in these publications that, together with α- and β-carotenes, CaCO3 is not suitable for its chromatography either: it will go through a CaCO3 column without retardation. From then on, Karrer used chromatography extensively for the clean-up of the crude extracts and the separation of the individual substances. This can be best illustrated by the number of publications of Kuhn and Karrer, listed in the bibliography section of the second edition of the chromatography book of Zechmeister and Cholnoky (23): for the period up to the summer of 1938 there are 52 papers by Kuhn and 49 papers by Karrer.
In the 1930s Karrer also isolated a number of other carotenoid compounds, establishing their structures. He also confirmed the structure of ascorbic acid (vitamin C) and other vitamins. For these achievements he received in 1937 the Nobel Prize in Chemistry. In the next decades, he extended his activities into other complex organic compounds, establishing their structure and developing their total synthesis. During his professional career Karrer authored or coauthored over 1000 publications and directed the work of over 200 Ph.D. students.
Karrer became one of the best known and respected chemists in Europe: his major contribution to the evolution of chromatography was his unequivocal proof that it is superior to all classical techniques used up to then for the isolation of pure substances. At the beginning of this report I already cited his 1939 statement; to this we should add one paragraph from his plenary lecture at the 1947 Congress of the International Union of Pure and Applied Chemistry, in which he clearly characterized Tswett's invention as one of the key developments of the 20th century (24):
No other discovery has exerted as great an influence and widened the field of investigation of the organic chemist as much as Tswett's chromatographic adsorption analysis. Research in the field of vitamins, hormones, carotenoids, and numerous other natural compounds would never have progressed so rapidly and achieved such a great results if it had not been for this new method.
Activities of László Zechmeister
Besides Kuhn and Karrer, the third scientist pioneering in carotenoid research in the 1930s, and having a major role in the rapid expansion of the use of chromatography was Zechmeister, professor at the University of Pécs, in Hungary. He also was the author of the first comprehensive chromatography textbook.
László Zechmeister (1889–1972) (Figure 5) was born in Gyoýr, a Hungarian city on the Danube, and studied at the Swiss Federal Technical University (E.T.H.) in Zurich, graduating in 1911 as a chemical engineer. He continued his graduate studies there under Richard Willstätter; his doctorate thesis dealt with investigations of the cellulose and lignin of trees. When in 1912 Willstätter moved from Zurich to Berlin-Dahlem, as director of the Kaiser Wilhelm Institute for Chemistry, he invited Zechmeister to join him as his assistant. However, at the outbreak of World War I, Zechmeister had to enlist in the Hungarian Army; soon he became a prisoner of war on the Russian front, returning to Hungary only in 1919. By that time there was a chaos in both Hungary and Germany and he could only find temporary positions. Finally, in 1923, he was offered professorship in Hungary, in a newly established university in the city of Pécs, in the southern part of the country, as the director of the Chemistry Institute within the Faculty of Medicine.
Figure 5
On paper this was a great honor to Zechmeister. He was only 33 years old and in Hungary such a young person never received a cathedra. However, the task he accepted was formidable: he had to start from scratch. Buildings had to be erected, laboratories established and equipped, a staff hired, and the country was very poor. We cannot compare Zechmeister's situation with Kuhn in Heidelberg, or Karrer in Zurich, who had generous funds, well-equipped laboratories and a large staff. In contrast, there was only one associate professor position in Zechmeister's new institute — László Cholnoky (1899–1967), his close collaborator — and a maximum of two or three graduate students.
In Pécs Zechmeister could finally start laboratory investigations only in the second half of the 1920s. Soon, he also entered the field of carotenoids and his particular interest was the investigation of the pigments of Hungarian red paprika: he published more than a dozen papers on this subject, the first in 1927, and the last in 1937. Zechmeister was considered such an expert on carotenoids that he was asked to contribute a chapter on these compounds for the Handbook of Plant Analysis published in 1932 (25), which he then expanded into a 340-page monograph published two years later (26).
It is difficult to establish when Zechmeister actually started to use chromatography. In his book chapter on carotenoids published in 1932 (25), Zechmeister already discussed in detail the chromatographic technique and its possible use for plant pigment investigations, and from the text of this chapter it is clear that he already had some experience in its use; however, his first journal paper specifically mentioning the use of chromatography was published only in 1934 (27). A typical chromatogram of Hungarian red paprika extract is shown in Figure 6. (Here Zechmeister used a composite column, with the addition of a layer of Ca(OH)2 to CaCO3. It had been shown by Karrer (28) that for the separation of the carotenes, Ca(OH)2 is also a suitable adsorbent.)

Figure 6
Most likely the main reason that Zechmeister started to publish on the use of chromatography later than Kuhn and Karrer was a shortage of instrumentation that would have been needed to gather all the data needed for a full publication. However, from 1934 on, Zechmeister continuously published a large number of papers on the use of chromatography: the bibliography section of the second edition of his book (23) lists 33 titles published by him before the summer of 1938.
Zechmeister's most important contribution to chromatography was his monograph on the "chromatographic adsorption method" (23). This was the right book, published at the right time, and it became an instant bestseller: the original edition published in 1937 was followed within a year by a greatly enlarged, second edition. About one-third of the text dealt with fundamentals and methodology, while two-thirds discussed applications, and a complete bibliography of papers describing the use of chromatography was added.
By the end of the 1930s Zechmeister was well established and respected both at home and abroad: he received a number of major awards, and had been invited a number of times to lecture in various universities, both in European countries and the United States. However, political events interrupted his life: the advent of Nazi dominance in Europe made for him intolerable to remain in Hungary, and he accepted an invitation to join the faculty of the California Institute of Technology, in Pasadena. He sailed to the United States in February 1940, with the last ship from an Italian port.
In Pasadena, Zechmeister remained active in the study of complex natural substances and the use of (classical) chromatography. In 1948, he finished the continuation of his chromatography textbook and it was published in 1950 (29). In the last decade of his active life, he concentrated on the chromatographic separation of stereoisomers: his last publication, a monograph on cis and trans isomeric carotenoids (30), was published in 1962, after his retirement (31).
Methodology
The chromatographic systems used in the laboratories of Kuhn, Karrer and Zechmeister were functionally similar to the original set-up of Tswett: glass tubes of about 15–20 cm long, with 1–3 cm diameter, were filled with an adsorbent and installed in standard laboratory filtering flasks (Figure 7). For semi-preparative purposes larger and wider column tubes also were used: we already have mentioned that Lederer used tubes up to a diameter of 7 cm, and Karrer sometimes also worked with 80-cm long columns. The sample solution was added to the top of the packing and then further developed by additional solvent volumes. During development, the sample components moved downward the column and slowly separated into (colored) rings of the pure compounds. When separation was completed the column packing was pushed slowly out of the tube and the colored rings were carefully physically separated from each other and from the rest of the packing. The individual compounds adsorbed in these separated rings were dissolved and sometimes rechromatographed again, using a different solvent.

Figure 7
An innovation introduced practically simultaneously by both the Heidelberg (32) and Zurich (33) groups was the chromatography of colorless substances, utilizing the fluorescence of the compounds: the separated compounds were observed under a fluorescence light. For this, chromatography had to be carried out in a tube of quartz and not glass. Karrer even gave a name to this variant: he called it ultrachromatography.
Because chromatography was interrupted while the separated compounds were still on the column, one column could be used only once. Therefore, usually a large number of column tubes were used in a laboratory; Zechmeister and Cholnoky have in their book (23) a photo of a rack holding dozens of such standardized tubes ready for use. Methodology was slowly changed in the second part of the 1930s, by continuing column development with additional solvent volumes, until finally the individual rings were washed out of the column with the solvent and collected as individual fractions. This variant was called Durchflusschromatogramm (flow-through chromatogram) or flüssiges Chromatogramm (liquid chromatogram). In the literature, this technique is usually credited to Koschara, with reference to his 1936 paper (34), however, it is definitely older. Tswett, in his 1910 book already suggested such an operation, and we have, for example, found a paper from 1935 by the group of Harold Cassidy at Yale University reporting on the use of this technique (35). The next step was then to use a detector at column's end, continuously monitoring the column effluent, as first described by Arne Tiselius in Uppsala, Sweden, in the early 1940s. However, this is another story.
An Appraisal
As we have discussed in the introduction, only very few scientists utilized chromatography in the first 25 years following the fundamental work of Tswett: we know only about half-a-dozen such laboratories, and the number of publications from these places is very limited. At the same time, however, the situation dramatically changed in 1930–1931: this is best demonstrated by the bibliography section of the chromatography textbook of Zechmeister and Cholnoky, listing over 500 publications up to the summer of 1938.
It is difficult to establish the reason for this contrast. Was it because Tswett published in an (albeit, widely read) botanical, and not chemical, journal, or because his major book was published in Russian? Or were Kuhn, Karrer, and Zechmeister in the 1930s more trustworthy than Tswett in 1906–1914? However, the more logical explanation for the sudden meteoric rise of chromatography is a change in the emphasis of scientific research. At the beginning of the 20th century the keywords in chemical (and biochemical) research were still isolation and purification: isolation from the accompanying material and purification from trace impurities, and this was done by extraction and crystallization. There was no interest in all the compounds present, one only wanted to isolate a few key compounds, and one had to work with large amounts. This attitude changed around 1930, and this change in the philosophy of research was best expressed in a lecture by G.M. Schwab, professor at the University of Munich (36):
Only after biochemistry, pressed by new problems, demanded methods for the reliable separation of small quantities of similar substances, could chromatography celebrate a rapid and brilliant resurrection.
The decade following the rebirth of chromatography in 1930–1931 can be considered as a true milestone period of the technique: its methodology was settled, the technique was universally accepted, and objections questioning the purity of fractions separated by chromatography were unequivocally confuted. Without any question, this was the particular merit of Richard Kuhn, Paul Karrer, László Zechmeister, and their associates. We, present-day chromatographers, all benefit from their fundamental work 70–75 years ago, widely opening the gate for the universal acceptance of chromatography.
Leslie S. Ettre
From 1988 to 2004, "Milestones in Chromatography" editor Leslie S. Ettre was associated with the Chemical Engineering Department of Yale University (New Haven, Connecticut), first as an adjunct professor and then as a research fellow. Previously, he had been with the Perkin-Elmer Corporation for 30 years. He is currently a member of LCGC's editorial advisory board. Direct correspondence about this column to "Milestones in Chromatography,"LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com.
References
(1) L.S. Ettre, LCGC 21(5), 457–467 (2003).
(2) F.M. Schertz, Plant Physiol. 4, 337–348 (1929).
(3) P. Karrer, Helv. Chim. Acta 22, 1149–1150 (1939).
(4) R. Willstätter and W. Mieg, Ann. Chem. 355, 1–28 1907).
(5) R. Willstätter and H.H. Escher, Z. Physiol. Chem. 76, 214–225 (1912).
(6) M.S. Tswett, Khromofilly v Rastitel'nom I Zhivotnom Mire (Chromophylls in the Plant and Animal World) (Karbasnikov Publishers, Warsaw, 1910).
(7) M. Tswett, Ber. Dtsch. Botan. Ges. 29, 630–636 (1911).
(8) R. Willstätter and A. Stoll, Untersuchungen über Chlorophyll: Methoden und Ergebnisse (Springer Verlag, Berlin, 1913), pp. 234-235.
(9) L.S. Palmer, Carotinoids and Related Pigments — The Chromolipids (Chemical Catalog Co., New York, 1922).
(10) B.v. Euler, H.v. Euler, and H. Hellström, Biochem. Z. 203, 370–384 (1928).
(11) T. Moore, Biochem. J. 24, 692–702 (1930).
(12) P. Karrer, J. Salomon, and H. Wehrli, Helv. Chim. Acta 12, 790–792 (1929).
(13) E. Lederer, J. Chromatogr. 73, 361–366 (1972).
(14) E. Lederer, In: 75 Years of Chromatography — A Historical Dialogue, L.S. Ettre and A. Zlatkis, Eds. (Elsevier, Amsterdam, 1979), pp. 237–245.
(15) R. Willstätter, Untersuchungen über Enzyme, (Springer Verlag, Berlin, 1928), Vol.I, p. 295.
(16) R. Kuhn and E. Lederer, Naturwiss. 19, 306 (1931).
(17) R. Kuhn, A. Winterstein, and E. Lederer, Z. Physiol. Chem. 197, 141–160 (1931).
(18) R. Kuhn and E. Lederer, Ber. Dtsch. Chem. Ges. 64, 1349–1357 (1931).
(19) Nachrichten aus Chemie und Technique 12, 286 (1964).
(20) A.J.P. Martin in Gas Chromatography in Biology und Medicine (1969 CIBA Foundation Symposium), R. Porter, Ed. (Churchill, London, 1969), p. 240.
(21) P.Karrer, R. Morf, and K. Schöpp, Helv. Chim. Acta 14, 1036–1046, 1431–1436 (1931).
(22) P. Karrer, K. Schöpp, and R. Morf, Helv. Chim. Acta 15, 1158–1165 (1932).
(23) L. Zechmeister and L. Cholnoky, Die Chromatographische Adsorptionsmethode (Springer Verlag, Wien), 1937; 2nd enlarged edition: 1938.
(24) E. Lederer and M. Lederer, Chromatography - A Review of Principles and Applications (Elsevier, Amsterdam, 1955), preface.
(25) L. Zechmeister in Handbuch der Pflanzenanalyse, G. Klein, Ed. (Springer Verlag, Vienna, 1932), Vol.3, pp. 1239-1350.
(26) L. Zechmeister, Carotinoide: Ein biochemi-scher Bericht über pflanzliche und tierische Polyenfarbstoffe (Springer Verlag, Berlin, 1934).
(27) L. Zechmeister and L. Cholnoky, Ann. Chem. 509, 269-287 (1934).
(28) P. Karrer and R. Morf, Helv. Chim. Acta 16, 625-641 (1933).
(29) L. Zechmeister, Progress in Chromatography 1938-1947 (J. Wiley & Sons, New York, 1950).
(30) L. Zechmeister, Cis-trans Isomeric Carotenoids, Vitamin A and Aryl Polyenes (Springer Verlag, Vienna, 1962).
(31) L.S. Ettre, Anal. Chem. 61, 1315A-1322A (1989); 62, 71A (1990).
(32) A. Winterstein and K. Schön, Z. Physiol. Chem. 230, 139–145 (1934).
(33) P. Karrer and K. Schöpp, Helv. Chim. Acta 17, 693 (1934).
(34) W. Koschara, Z. Physiol. Chem. 239, 89-96 (1936).
(35) H.N. Holmes, H. Cassidy, R.S. Manly, and E.R. Hartzler, J. Am. Chem. Soc. 7, 1990–1993 (1935).
(36) G.M. Schwab and K. Jockers, Angew. Chem. 50, 546-553 (1937).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










