Readers' Questions
Questions from the e-mail bag are considered in this month's instalment.
Questions from the e-mail bag are considered this month.
One aspect I enjoy about being the "LC Troubleshooting" editor is getting to interact with readers through a wide variety of liquid chromatography (LC) questions that I get via e-mail. This month I'll share some of the more interesting ones I've received recently. If you have a question for me, feel free to contact me at the e-mail address listed at the end of this article.
Acceptable Retention
Reader: I've heard you say that the retention factor, k, should not be less than 2 for an isocratic method. Is this a hard-and-fast rule? I'm having trouble getting the first peak retained and would like to have a fast run.
JWD: As a general rule, a retention factor in the range 2 < k < 10 will give you the "best" chromatography, but this is no guarantee of the best separation. Also, some samples have such a wide polarity range that you can't fit them in this retention window. In such cases, 1 < k < 20 certainly is acceptable. When even this extended range of k-values is not possible, you should seriously consider gradient elution instead of an isocratic method.
Let's review why we set these k-value guidelines. First, recall that the retention factor is calculated as

where tR is the retention time and t0 is the column dead time, usually determined by the first rise in the baseline at the "solvent front." Resolution is a function of k/(1 + k), so if we plot retention as the retention factor on the x-axis and resolution as k/(1 + k) on the y-axis, we see a plot like that of Figure 1. You can see that the resolution line starts out at a very low value and rises to a plateau as k increases. This relationship is the basis of the recommendations for k-ranges for isocratic methods. When 2 < k < 10, you can see that the plot begins to flatten out, but run times aren't excessive. In this region small changes in k will result in very small changes in resolution. Or another way of looking at this is that the method is robust to small changes in variables that might change retention, such as the percentage of organic solvent in the mobile phase, temperature or pH. On the other hand, if we extend the acceptable k-range to 1 < k < 20, the early peaks lie on a much steeper portion of the curve. This means that the same change in k that caused little concern with longer retention times will cause larger changes in resolution. Thus, methods with k < 2 tend to be less stable. Another problem with peaks with k < 2, and certainly k < 1, is that there is more likelihood of interferences from unretained material at t0. I've also plotted the run time and peak height in Figure 1. As k increases, run time increases and peaks broaden and are shorter; both of these are undesirable, so smaller k-values for the last peak are desirable.
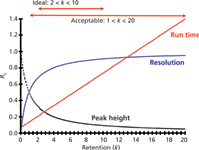
Figure 1: A plot of resolution, expressed as k/(1 + k) vs. retention (k). The effect of retention on peak height and run time are also shown.
However, it must be acknowledged that the recommendations of k-ranges shown in Figure 1 are just that, recommendations, not hard-and-fast rules; there will always be exceptions. For example, sometimes it is not possible to get sufficient retention of a very polar peak so that k > 1 can be obtained. Or for very clean samples, the baseline disturbance at t0 may be small enough that k = 0.5 provides acceptable separation for adequate quantification. But when we make a decision to develop and validate a method with such small retention, we should go into it with our eyes open and recognize the potential problems.
What are some alternatives? If run time is your major concern, it may be possible to increase k-values so that the first peak has k > 2, then to increase the flow rate and reduce the retention time, because k is not affected by flow rate. Or if retention on a conventional C18 column is too short for a polar compound, maybe an embedded polar phase column will provide an acceptable alternative. Another alternative might be to use hydrophobic interaction chromatography (HILIC), which is a form of normal-phase chromatography. With HILIC, retention orders typically are the opposite of those obtained using reversed-phase chromatography, so polar compounds are well retained and nonpolar ones come out early.
New or Used Column?
Reader: Should I start my method validation experiments with a new column? I've heard some people say this is mandatory, but I don't see why I can't continue with the column I have been using — after all, it still works.
JWD: I don't think it is necessary to always put a new column in when validation commences, but I do strongly recommend that the influence of the column should be investigated as part of the validation experiments. One of the checks of precision that is often made is called intermediate precision, which refers to changes in conditions that are not easy to quantitatively control. For example, changes to the mobile-phase composition or column temperature can be quantitative, and fall in the category of repeatability. Changes resulting from different operators, different equipment and different columns are things that we can identify as changes, but are more qualitative than quantitative changes — these are the intermediate precision items that are tested.
Traditionally, intermediate precision includes checking the results for three different columns — two from one batch of packing material and one from another batch. I think such checks are of less importance today with the high degree of reproducibility achieved by column manufacturers for modern columns. Perhaps a more important check is to compare results between a new column and a well-used column. So in your case, it would be smart to check both your used column and a new one to be sure the same analytical results could be obtained.
One caution is appropriate when a used column is included in your validation experiments: You want to be sure that the used column accurately reflects the chemistry of a used column under normal application of the method. If the used column has been operated under a wide variety of conditions during method development or used with another method, and especially if any of the experiments were outside the 2 < pH < 8 range where most silicabased columns are stable, you may have inadvertently changed the column chemistry. However, if a new column was installed at the time you began your final prevalidation experiments for the method, where you perform a mini-validation to be sure the method is sufficiently stable to pass validation, the column ageing process is more likely to reflect what a column would experience in real life.
So the bottom line here is that you should check the performance of your method with more than one column, and selecting an appropriately used column as one of the test columns seems reasonable to me. However, interpretation of regulatory guidelines differs widely, so rather than take my word as gospel, I'd suggest you seek advice from your quality unit, as well.
Setting Limits for Herbal Products
Reader: I work for a company that "manufactures" herbal materials that are sold to clients who formulate these into products that are sold to the public. As a supplier, we need to ensure that the material has the appropriate potency, so I need to set acceptance limits for my LC methods. How do I go about that?
JWD: As you know, herbals are not regulated as strictly as traditional pharmaceutical products, and the performance criteria for pharmaceutical methods may not be appropriate to apply to your raw materials. However, the general principles that are used for pharmaceuticals can be used as guidelines.
First, you need to start with the product specifications your company quotes to clients. For example, herbal material X contains 50–100 mg/kg of active ingredient Y. This means that you need to show with some degree of confidence that X contains 75 ± 25 mg/kg of Y. Next, you need to decide how often it is acceptable to ship product outside this range — for example, if 95% of the time you want to comply with the target range, this would correspond to 4 standard deviations (SD), so your passing material would have to be 75 ± 12.5 mg/kg (1 SD). This would correspond to a relative standard deviation (RSD) of 12.5/75 = 17% RSD. To have confidence reporting 17% RSD, you probably want your method to perform at half this level of imprecision or better. So developing a method that has an imprecision of 5–10% may be adequate. The regulations for this are pretty vague, but you need to develop a test process that is scientifically sound and defensible. Finally, your decisions should be influenced by any safety risk, such as toxicity, that might be involved if analytical errors are made.
Peak Purity
Reader: One of the peaks in my samples tails a bit, and I think it may be an impurity that is not separated from the peak. Will the diode-array detector's peak-purity output show me if the peak is pure or not?
JWD: This is one of those questions that gets answered with a "maybe." The peak-purity determination is made by comparing UV spectra taken at different points across a peak. If the spectra are the same, the peak is considered pure, whereas if the spectra are different, the presence of an impurity is indicated. This is all well and good in principle, and I have read convincing articles showing the utility of this measurement. However, in actual practice, I have found that most users don't provide such glowing praise. I think this has to do with the challenge of the measurement. Often if the two peaks have similar retention times, the structures are similar, which means that the UV spectra are also likely to be similar. Many compounds do not show much UV absorbance other than the end-absorbance characteristic of most organic compounds in the < 210 nm region, so this may further compromise spectral comparison. Finally, if a small peak is eluted on the tail of a large one, there may be sufficient difference in peak size that even if there are small differences in the spectra, they will not be of sufficient magnitude to definitively show up in the peakpurity calculations. These problems are likely the source of the rather mediocre endorsement of peakpurity measurements by most users.
On the other hand, there is plenty of literature supporting peak purity measurements, and if the spectra of the two compounds are sufficiently different and there is enough of the minor component present, the peakpurity calculations may indeed indicate the presence of a second compound. My advice is to try the peak-purity measurement and see what happens. Just remember that it may be possible to show that a peak is impure by using peak-purity or mass-spectral measurements, but it is not possible to prove that a peak is pure.
Degassing
Reader: I have been using sonication to degas my mobile phase, but recently I was told that this is not effective. Can you clarify this?
JWD: Today most LC systems include an in-line vacuum degasser, so issues with mobile-phase degassing, which once were at the top of the list of common LC problems, are largely a thing of the past. However, there are many LC systems, such as yours, that are still in use and do not have an automatic degasser installed. For years, sparging the mobile phase with helium was the gold standard for degassing, and this is still the most effective way to remove air from the mobile phase. Another popular technique that has been used for years is vacuum degassing of the bulk solution. Vacuum degassing while simultaneously sonicating the solution is considered by many users to be superior to vacuum degassing alone, but I have never seen a well-executed study comparing the two techniques. In one study I read, helium sparging removed about 80% of the dissolved air and vacuum degassing about 60%. However, sonication alone was only about 30% effective, so it is not very promising.
Another consideration is that different pumping system designs have different levels of tolerance for dissolved gas in the mobile phase. At the extremes of systems that I have used, I remember one LC system that required simultaneous helium sparging and a positive head pressure on the mobile-phase reservoir to avoid bubble problems in the pump. In the same laboratory we had another brand of pump that was so tolerant of air that it would prime itself if a dry inlet tube was dropped into a reservoir. In general, high-pressuremixing systems are more tolerant of dissolved gas than LC systems that use low-pressure mixing. My conclusion is that if you are using a high-pressuremixing, bubbletolerant system, then sonication may be adequate, but for other systems, sonication is unlikely to provide sufficient degassing for reliable operation. Try it and see — you may be lucky!
John W. Dolan is vice president of LC Resources, Walnut Creek, California, USA. He is also a member of LCGC Europe's editorial advisory board. Direct correspondence about this column should go to "LC Troubleshooting", LCGC Europe, 4A Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK, or e-mail the editor, Alasdair Matheson, at amatheson@advanstar.com

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.









