Rapid UHPLC Method Development for Omeprazole Analysis in a Quality-by-Design Framework and Transfer to HPLC Using Chromatographic Modelling
LCGC Europe
This article describes ways to apply quality-by-design principles to build in a more scientific and risk-based multifactorial strategy in the development of an ultrahigh-pressure liquid chromatography (UHPLC) method for omeprazole and its related impurities.
The aim of this study was to apply quality-by-design principles to build in a more scientific and risk-based multifactorial strategy in the development of an ultrahigh-pressure liquid chromatography (UHPLC) method for omeprazole and its related impurities.
The quality-by-design concept was outlined years ago by Joseph M. Juran (1) and is used in many industries to improve the quality of products and services simply by planning quality from the beginning. Since the US Food and Drug Administration (FDA) announced its "Pharmaceutical Current Good Manufacturing Practices (cGMPs) for the 21st Century" initiative (2) in 2002, a quality-by-design approach has also been sought in the pharmaceutical industry.

Through the International Conference on Harmonization (ICH), this concept resulted in ICH guideline Q8(R2) in which quality-by-design is defined as "a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management" (3).
Although ICH guideline Q8(R2) doesn't explicitly take analytical method development into account and no other regulatory guideline has been issued, the quality-by-design concept can be extended to a systematic approach that includes the definition of the methods goal, risk assessment, design of experiments, developing a design space, verification of the design space, implementing a control strategy, and continual improvement to increase method robustness and knowledge (4). The novelty and opportunity in this approach is that working within the design space of a specific method can be seen as an adjustment and not a postapproval change (4).

Photo Credit: Image Source/Getty Images
A systematic approach should replace the still common "screening", also known as a trial-and-error approach, in which one factor at a time (OFAT) is varied until the best method is found. The OFAT approach is time-consuming and often results in a nonrobust method because interactions between factors are not considered.
Today, systematic concepts use experimental design plans as an efficient and fast tool for method development. In a full or fractional factorial design, a couple of experiments are carried out in which one or more factors are changed at the same time. By using statistical software tools (for example, Design Expert from Stat-Ease, Inc.), the effect of each factor on the separation can be calculated and the data can be used to find the optimum separation (4). In our laboratory, this concept is used when the development of nonchromatographic methods is necessary.
However, the easiest and fastest way of developing a liquid chromatographic method is by using chromatography modelling, especially in combination with ultrahigh-pressure liquid chromatography (UHPLC) technology. Based on a small number of experiments, these software applications can predict the movement of peaks when parameters such as eluent composition or pH, flow rate, column temperature, column dimensions, and particle size are changed (5–11). When necessary, the developed method can be transferred (back) to high performance liquid chromatography (HPLC).
In our laboratory we have been using visual chromatographic modelling (software packages) for many years now in HPLC and UHPLC method development and it has resulted in very robust methods (4,12–14). The aim of this study was to apply quality-by-design principles to build in a more scientific and risk-based, multifactorial strategy in the development of a new UHPLC method for testing the purity of omeprazole.
Omeprazole belongs to the group of proton-pump inhibitors and is one of the most widely prescribed drugs. It suppresses gastric acid secretion by specific inhibition of the enzyme hydrogen-potassium adenosine triphosphatase (H+, K +–ATPase). Omeprazole formulations are used to treat acid reflux, heartburn, ulcer disease, and gastritis (15).
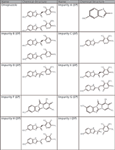
Figure 1: Chemical structures of omeprazole and its related impurities.
Omeprazole is described in the monograph of the European Pharmacopeia (EP) (16). Purity testing for omeprazole is accomplished by using HPLC with UV detection on a 125 mm × 4.6 mm, 5-µm dp C8 column in isocratic mode with an eluent consisting of 27 vol% acetonitrile and 73 vol% disodium hydrogen phosphate solution (pH 7.6) and a flow rate of 1.0 mL/min. On the basis of the synthetic route, the monograph recommends testing the impurities A, B, C, D, E, H, and I by HPLC, and the impurities F and G have to be tested by a photometric method (chemical structures are shown in Figure 1). A typical chromatogram of a selectivity standard solution containing omeprazole and its related impurities A–I obtained using the EP method is given in Figure 2 and shows that the method was developed without any chromatography knowledge. Some of the impurity peaks show coelution, but the last three peaks are separated from each other with a huge distance of 10 min each.
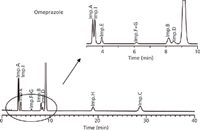
Figure 2: Typical chromatogram of a selectivity standard solution containing omeprazole and its related impurities AâI by using the purity method published in the European Pharmacopoeia. Column: 125 mm à 4.6 mm, 5-µm dp Symmetry C8 column; mode: isocratic; eluent: 27 vol% acetonitrile and 73 vol% disodium hydrogen phosphate (1.4 g/L), adjusted with phosphoric acid to pH 7.6; flow rate: 1 mL/min.
Several analytical procedures for the determination of omeprazole and its related impurities have been described. A review of the analytical methods for the determination of omeprazole, mostly in plasma and urine, was published in 2007 (17). Only some recent publications focus on stability-indicating methods for the analysis of impurities and degradation products in omeprazole formulations (18–20). As far as we know, no analytical method has been published that would separate all synthesis impurities and degradation products mentioned in the EP monograph. Therefore, there is a need for a simple, fast, and reliable purity method for the determination of omeprazole and its related impurities in the active pharmaceutical ingredient (API) and in pharmaceutical formulations.
Experimental
Chemicals: Methanol and acetonitrile were HPLC-gradient grade (Sigma). All other chemicals were at least analytical grade and were also purchased from Sigma. Ultrapure water was obtained using a TKA water purification system (Thermo Fisher Scientific).
Equipment and Chromatographic Conditions: For the UHPLC experiments, an Acquity UPLC H-class system consisting of a quaternary solvent system with a solvent-selection valve, a sample injection system, column management system, and a photodiode-array detector, all controlled by Empower 2 C/S-software (Waters) was used. The dwell volume of the system was 0.400 mL.
For the HPLC experiments an Alliance 2695 XE system with a model 2996 photodiode-array detector, controlled by Empower 2 C/S-software (Waters) was used. The dwell volume of the system was 1.000 mL.
A 50 mm × 2.1 mm, 1.7-µm dp Acquity UPLC BEH C18 column (Waters) was used in the UHPLC study and the equivalent 50 mm × 4.6 mm, 2.5-µm dp XBridge BEH C18 column (Waters) was used in the HPLC study.
All method development experiments were performed on the UHPLC system in gradient mode. Eluent A was 10 mM ammonium bicarbonate buffer at different pH values (adjusted with ammonia) and eluent B was acetonitrile. Eluent C was methanol (for screening experiments only). The flow rate was set to 0.7 mL/min and the injection volume was 2 µL.
The temperature in the experiments was optimized between 30 °C and 60 °C. The UV detection of the compounds of interest was carried out at 303 nm and the UV spectra were taken in the range of 200–400 nm.
Software: For chromatography modelling the DryLab 4.0 software package (Molnar-Institute) was used. The software package includes PeakMatch and 3-D-Robustness modules.
Standard Preparation: A selectivity standard solution containing 0.2 mg/mL omeprazole (in-house standard substance) and approximately 0.002 mg/mL of each of the nine impurities was prepared with a 2:8 (v/v) mixture of acetonitrile and 10 mM ammonium bicarbonate buffer as the solvent. The impurities A, B, C, E, H, and I were obtained from LGC. Impurity D was purchased from the European Directorate for the Quality of Medicines (EDQM) and the impurities F and G were obtained from the U.S. Pharmacopeial Convention (USP). The selectivity standard solution was protected from light by using amber glassware.
Results and Discussions
Development Strategy: Our development strategy (4) follows quality-by-design principles and can be divided into six steps as follows:
Step 1: Definition of Method Goals: Our primary goal was to develop a stability-indicating method that separates the API from all impurities with a critical resolution (Rs,crit) of no less than 2.0. To speed up the development process, UHPLC technology was used; the final method was intended to be transferred to HPLC.
Step 2: Risk Assessment: Using a fishbone diagram, an early risk assessment was identified and possible risk factors associated with sample preparation as well as the instrumental analysis were prioritized. The initial list of potential parameters that can affect critical quality attributes (CQAs) were ranked and prioritized using failure mode and effects analysis (FMEA).
It was obvious that resolution is a CQA and the selectivity term α in the general equation Rs = 0.25N1/2 [(α – 1)/α][k/(1 + k)] has the greatest impact on the resolution. Selectivity is influenced by the mobile phase composition, column chemistry, and temperature (21), and the influence should be investigated by design of experiments (DoE).
Other CQAs that were taken into account include the robustness of the method and the run time.
Step 3: Design of Experiments: For the critical process parameters (CPPs), which have an impact on the CQAs, experiments should be conducted to determine acceptable ranges. As the result of the risk assessment, the four parameters gradient time (tG), temperature (T), pH of the aqueous eluent A, and type of the organic eluent B were screened and optimized because of their strong known influential effects on selectivity.
A set of 12 experiments was performed for each of the two organic eluents methanol and acetonitrile under the following conditions: gradient times: tG1 = 3 min and tG2 = 9 min; temperatures: T1 = 30 °C and T2 = 60 °C. The pH values of the buffer were pH1: 8.0, pH2: 8.5, and pH3: 9.0. Because of prior knowledge, a modern C18 column was used.
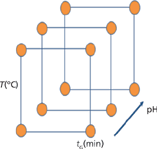
Figure 3: Graphical description of the design of experiments plan for the method development by using chromatographic modelling: For each organic eluent, methanol and acetonitrile, 12 experiments have to be performed with low and high values for T, tG, and pH.
The ranges between these factors were large enough to induce peak movements to discover hidden peaks (4). A graphical description of the DoE plan can be seen in Figure 3.
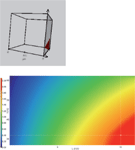
Figure 4: Three-dimensional resolution cube (tG/T/pH model) and the corresponding two-dimensional resolution map (tG/T model) at pH 9.0 for methanol as the organic solvent in the UHPLC gradient method. The red regions in the resolution maps represent the design space, in which the performance criteria are met.
Step 4: Design Space: The retention times of all peaks of interest in the 12 experiments were entered into the chromatographic modelling software and matched in each of the chromatograms by using the PeakMatch module.
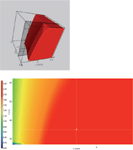
Figure 5: Three-dimensional resolution cube (tG/T/pH model) and the corresponding two-dimensional resolution map (tG/T model) at pH 8.75 for acetonitrile as the organic solvent in the UHPLC gradient method. The large red regions in the resolution maps represent the design space, in which performance criteria are met.
Based on the limited set of only 12 experiments, the modelling software builds a three-dimensional model of the critical resolution (the so-called "knowledge space"), in which the combined influence of the optimized parameters are visualized. The modelling software uses a colour code to represent the value of the critical resolution: Warm, "red" colours show large resolution values (Rs > 2.0), and cold, "blue" colours show low resolution values (Rs < 0.5) corresponding to regions of peak overlaps. The red geometric bodies within the knowledge space, in which the performance criteria are met, is called the design space. The ICH Q8 guideline defines the design space as follows (3):
"The multidimensional combination and interaction of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality. Working within the design space is not considered as a change. Movement out of the design space is considered to be a change and would normally initiate a regulatory post approval change process."
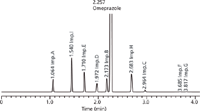
Figure 6: Predicted UHPLC chromatogram for omeprazole and its related impurities for conditions at the working point (for details see text).
Figures 4 and 5 show the three-dimensional resolution cubes for methanol and acetonitrile as the organic eluent in the UHPLC gradient method. A visual inspection shows that the design space in the methanol cube is much smaller than the design space in the acetonitrile cube. That means that the method with acetonitrile is more robust than the method with methanol and all the peaks in the chromatogram are well separated from each other (baseline resolution).
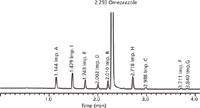
Figure 7: Experimental UHPLC chromatogram of omeprazole spiked with its related impurities AâI for conditions at the working point (for details see text).
Therefore, acetonitrile was chosen as the organic eluent and, from the corresponding design space, the working point was selected by visual examination. There are several possible alternative working points within the design space, but we looked for the highest critical resolution (Rs,crit) and best robustness of the method. This working point was found in the cube at tG 4.0 min, T 35 °C, and pH 8.75. The predicted and experimental chromatograms for this working point are shown in Figures 6 and 7.
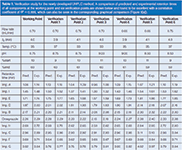
Table 1: Verification study for the newly developed UHPLC method. A comparison of predicted and experimental retention times of all components at the working point and six verification points are shown below and found to be excellent with a correlation coefficient of R2 = 0.999, which can also be seen in the corresponding graphical comparison (Figure 8[a]).
A verification study comparing predicted and experimental retention times for the working point and six verification points around the working point, but within the design space, was found to be excellent with a correlation coefficient of 0.999, as shown in Table 1 and Figure 8(a). This is also in compliance to previous reported data (4,22,23).
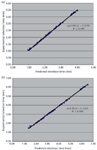
Figure 8: Plots of experimental retention time versus predicted retention time for (a) the UHPLC method and (b) after method transfer to HPLC.
An important part of our method development strategy is to perform robustness testing of the developed method before the validation study. The ICH guideline Q2 (R1) (24) defines robustness as follows:
"[. . .] the reliability of an analysis with respect to deliberate variations in method parameters. The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small, but deliberate variations in method parameters and provides an indication of its reliability during normal usage."
The robustness of the developed method was studied using the robustness module of the chromatographic modelling software. In a three-level, full-factorial design, the module used the previously constructed and verified design space for "in silico" robustness calculations (4). The six parameters tG (4 min ± 0.1 min), T (35 °C ± 2 °C), pH (8.75 ± 0.1), flow rate (0.7 mL/min ± 0.05 mL/min), and the %B start (10% ± 1%) and %B end (60% ± 1%) of the gradient were varied at three levels (+1, 0, –1).
Figure 9 shows the frequency of the distribution of the resolution values Rs,crit for all 729 experiments. It can be seen that the required resolution of 2.0 can be reached in all experiments. Therefore, the developed method is robust against small changes of chromatographic parameters.
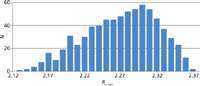
Figure 9: Frequency distribution of the Rs,crit values for all 729 experiments of the robustness study on the UHPLC system. The six parameters tG (4 min ± 0.1 min), T (35 °C ± 2 °C), pH (8.75 ± 0.1), flow rate (0.7 mL/min ± 0.05 mL/min), and the %B start (10% ± 1%) and %B end (60% ± 1%) of the gradient were varied at three levels (+1, 0, -1). All experiments fulfill the requirement for resolution Rs,crit no less than 2.0. That means that the failure rate is 0, so there will be no method-related out-of-specification (OOS) results and production quality control will be smooth and robust.
A formal validation study should be performed before this new method can replace the existing method.
Step 5: Method Control Strategy: The ICH Q8 guideline defines the control strategy as "a planned set of controls, derived from current product and process understanding that ensures process performance and product quality[. . .]" This means that the control strategy should be implemented to ensure that the developed method is performing as intended. Usually, this can be done by using a system suitability test. In our method development strategy, the resolution of the critical peak pair (Rs,crit), was chosen as a system suitability test parameter and should not be less than 2.0.
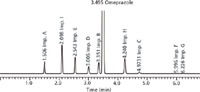
Figure 10: Predicted HPLC chromatogram for omeprazole and its related impurities for conditions after the transfer to the HPLC system (for details see text).
Step 6: Continual Improvement: In this last step further experiments can be planned and repeated to try out better columns and eluents to further adjust or improve the position of the working point. In addition, business needs — for example, the transfer of the developed UHPLC method (such as from the research and development [R&D] laboratory) to HPLC conditions (such as into the quality control [QC] laboratory) — can be taken into account.
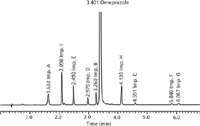
Figure 11: Experimental HPLC chromatogram of omeprazole spiked with its related impurities AâI for conditions after the transfer to the HPLC system (for details see text).
To transfer the UHPLC method to HPLC conditions, the changed column dimensions, particle sizes, and system dwell volumes were used to scale up the flow rate and gradient time. This can be made by using free available method transferring tools (such as the Acquity Columns Calculator from Waters). A smart way is to use the modelling software for the transfer and calculate the gradient time and flow rate. At the same time, the corresponding chromatograms can be visualized.
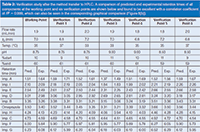
Table 2: Verification study after the method transfer to HPLC. A comparison of predicted and experimental retention times of all components at the working point and six verification points are shown below and found to be excellent with a correlation coefficient of R2 = 0.999, which can also be seen in the corresponding graphical comparison (Figure 8[b]).
Small adjustments of the scaled conditions for flow rate and gradient time had to be made to reduce the back pressure in the HPLC system. The predicted and experimental chromatograms for the up-scaled HPLC method can be seen in Figures 10 and 11. A second verification study for the working point on the HPLC system and six verification points around the working point confirmed the accuracy of the prediction (see Table 2 and the corresponding graph in Figure 8[b]). In addition, the robustness study after the transfer to the HPLC system shows that the failure rate is still zero (see Figure 12).
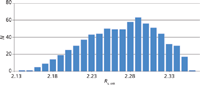
Figure 12: Frequency of the distribution of the resolution values Rs,crit for all 729 experiments of the robustness study after the transfer to the HPLC system. The six parameters tG (7 min ± 0.1 min), T (35 °C ± 2 °C), pH (8.75 ± 0.1), flow rate (1.9 mL/min ± 0.1 mL/min), and the %B start (10% ± 1%) and %B end (60% ± 1%) of the gradient were varied at three levels (+1, 0, -1). All experiments still fulfill the requirement for resolution Rs,crit of no less than 2.0. That means that the failure is also 0.
Table 3 summarizes the chromatographic parameters and tolerances of the final method.

Table 3: Description of the final analytical procedure including the tolerance limits.
Conclusions
A quality-by-design–based method development strategy for a method to test the purity of omeprazole has been presented here. The scientific and risk-based multifactorial method development strategy uses visual chromatographic modelling as a fast and easy to use development tool. To speed up the method development process, all experiments were performed on a UHPLC system. The final method was successfully transferred to HPLC conditions. Verification studies between predicted and experimental retention times confirm the accuracy of the chromatographic modelling process.
All experiments, from the planning, performing on the UHPLC system, verification and transfer to HPLC, to the reporting, were made within one week.
Alexander H. Schmidt is quality control director at Steiner Pharmaceuticals in Berlin, Germany. He is also head of analytical development of an R&D and contract analysis lab and supervises 35 lab assistants and chemists. Over the years, he has published numerous articles on HPLC and UHPLC method development for pharmaceuticals and complex natural compound mixtures. He is also a guest lecturer at the Beuth University of Applied Sciences, in Berlin, Germany. In addition, he is currently writing his doctoral thesis at the Institute of Pharmacy at Freie Universität Berlin in Germany.
Mijo Stanic joined the development team at Steiner Pharmaceuticals as a lab assistant and was promoted to deputy lab manager in early 2013.
References
(1) J.M. Juran, Juran on Quality by Design: The New Steps for Planning Quality into Goods and Services (The Free Press, New York, USA, 1992).
(4) A.H. Schmidt and I. Molnár, J. Pharm. Biomed. Anal. 78–79, 65–74 (2013).
(5) L.R. Snyder and J.L. Glajch, Computer-assisted Method Development for High Performance Liquid Chromatography, (Elsevier, Amsterdam, The Netherlands, 1990).
(6) L.R. Snyder and J.L. Glajch, J. Chromatogr. A 485, 1–675 (1989).
(7) I. Molnár, J. Chromatogr. A 965, 175–194 (2002).
(8) I. Molnár, H.-J. Rieger, and K.E. Monks, J. Chromatogr. A 1217, 3193–3200 (2010).
(9) I. Molnár and K.E. Monks, Chromatographia 73 (Suppl.1), 5–14 (2011).
(10) K. Jayaraman, A.J. Alexander, Y. Hu, and F.P. Tomasella, Anal. Chim. Acta 696, 116–124 (2011).
(11) K. Monks, I. Molnár, H.-J. Rieger, B. Bogáti, and E. Szabó, J. Chromatogr. A 1232, 218–230 (2012).
(12) A.H. Schmidt and I. Molnár, J. Chromatogr. 948, 51–63 (2002).
(13) A.H. Schmidt, J. Liq. Chromatogr. Relat. Technol. 28, 871–881 (2005).
(14) A.H. Schmidt, M. Stanic, and I. Molnár, J. Pharm. Biomed. Anal. 91, 97–107 (2014).
(15) Commentary of the European Pharmacopoeia (in German), 38 supplement, Deutscher Apotheker Verlag, Stuttgart, Germany, (2011).
(16) "Monograph Omeprazole" in the European Pharmacopoeia, Seventh ed. (Deutscher Apotheker Verlag, Stuttgart, Germany, 2011).
(17) M. Espinosa Bosch, A.J. Ruiz Sanchez, F. Sanchez Rojas, and C. Bosch Ojeda, J. Pharm. Biomed. Anal. 44, 831–844 (2007).
(18) C. Iuga, M. Bojita, and S.E. Leucuta, Farmacia 57, 534–541 (2009).
(19) K.B. Borges, A.J.M. Sanchez, M.T. Pupo, P.S. Bonato, and I.G. Collado, J. AOAC Int. 93, 1811–1820 (2010).
(20) P. Venkata Rao, Ch.K. Sanjeeva Reddy, M. Ravi Kumar, and Danta Durga Rao, J. Liq. Chromatogr. Relat. Technol. 35, 2322–2332 (2012).
(21) L.R. Snyder, J.J. Kirkland, and J.L. Glajch, Practical HPLC Method Development, 2nd ed. (Wiley-Interscience, New York, USA, 1997).
(22) M.R. Euerby, G. Schad, H.-J. Rieger, and I. Molnár, Chromatogr. Today 3, 13–20 (2010).
(23) K.E. Monks, H.-J. Rieger, and I. Molnár, J. Pharm. Biomed. Anal. 56, 874–879 (2011).

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.










