Avoiding Reversed-Phase Chromatography Problems Through Informed Method Development Practices: Choosing the Stationary-Phase Chemistry
LCGC Europe
Many problems encountered executing HPLC methods are a result of decisions made during early method development. This article discusses one critical variable in method development, the choice of stationary phase chemistry.
Many problems encountered executing high performance liquid chromatography (HPLC) methods are the result of decisions made during early method development. This instalment of "Column Watch" discusses a critical variable in method development: the choice of stationary-phase chemistry. We discuss two stationary phase classes, embedded polar group (EPG) and perfluorophenyl (PFP), that are highly complementary to alkyl phases from a fundamental molecular interaction point of view. An understanding of the contrasting interactions that these different classes of stationary phase chemistries provide then leads to more accurate decisions regarding what phases may be most appropriate for a given set of analytes.
In practice, most chromatographers will initially reach for their favourite C18 column when commencing method development (1). An analyst will possibly evaluate a column using a scouting gradient, adjusting parameters such as organic percentage, pH, and organic modifier type until the desired retention and selectivity are obtained. In cases where the desired results are not obtained, many scientists will then choose their second favourite C18 column and repeat this process. If the practice is unsuccessful, it becomes clear that use of a C18 stationary phase will not provide the necessary separation and the analyst is then faced with a choice: change the stationary phase or force the C18 phase to do something it does not inherently do.
Forcing a C18 phase to provide interactions that are not inherent to a significant degree, such as ion exchange, can be induced by such means as the addition of ion-pairing reagents. Although ion-pair chromatography usually works, it often comes at a significant price. Because of the complexity of the interactions, ion-pairing methods tend to be difficult to reproduce, transfer, and troubleshoot (2). C18 systems can also be "made" to work by moving to highly complex mobile phases or extreme temperatures and pH levels. Again, such methods tend to lack robustness and ruggedness, causing extensive headaches later in the method lifetime.
A preferred approach is to use alternative stationary phases to obtain the desired retention and selectivity. This approach is not often chosen because of a lack of knowledge of interactions provided by the plethora of stationary phases available from many manufacturers. The objective of this work is to compare and contrast interactions provided by two main classes of alternative stationary phases to commonly used alkyl phases; namely, polar-embedded phases and fluorinated aromatic phases. By understanding the interactions that are available on such column chemistries, method development analysts can readily identify phases most appropriate for a given sample. The use of the correct tools facilitates method development and validation. In addition, using the right column generates an increase in method robustness and ruggedness because of the simpler mobile phases that are generally required.
Molecular Interactions
Resolution in any chromatographic system is dependent on efficiency, retention, and especially selectivity. Although a lot of attention has been paid to efficiency in recent years (for instance, sub-2-µm and superficially porous particles), retention and selectivity are of equal or greater importance and are dependent on the inherent chemistry of the stationary phase. For example, if a classic fully porous C18 phase is not providing any resolution of a given critical pair, changing to a sub-2-µm or superficially porous C18 is not likely to provide the desired separation. If a change in selectivity is required, a different set of interactions needs to be invoked to better delineate the differences between the solutes. Interactions that contribute to retention and selectivity can be divided into three main categories: dispersive interactions, polar interactions, and ionic interactions. Dispersive interactions are transient electronic interactions such as van der Waals forces or London forces. These forces are relatively weak on their own, but collectively are highly responsible for solubility and, thus, partitioning between mobile and stationary phases. Polar interactions such as the various dipole interactions are stronger than dispersive forces and also provide spatial selectivity. For example, if the stationary phase donates a hydrogen atom towards a hydrogen bond, the receiving analyte must be able to accept that hydrogen in a certain direction or range of directions. Such a system provides increased selectivity, not only from analytes that exhibit the ability to accept the hydrogen bond, but also analytes that differ in the spatial arrangement of the hydrogen bond donors. Full-charge interactions or ionic interactions provide very strong, relatively long-range interactions and represent the third class of mechanisms.
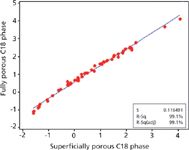
Figure 1: Plot of log k versus log k comparing fully porous C18 and superficially porous C18 phases. Fully porous phase column: 10 cm à 2.1 mm, 1.9-µm dp Titan C18; superficially porous phase column: 10 cm à 2.1 mm, 2.7-µm Ascentis Express C18; mobile phase: 10 mM ammonium acetate (70:30 water:acetonitrile) adjusted to pH 4 with formic acid; flow rate: 0.3 mL/min; column temperature: 35 °C; injection volume: 2 µL; detection: MS; mode: ESI+; scan range: 100â1000 m/z. 60 component mixture of analytes at 300 ng/mL varying in Log P and pKa values.
Generally, modern C18 stationary phases provide similar interactions with few exceptions. The traditional approach of method development for using different C18 columns stems from earlier days when C18 columns from different manufacturers or brands were significantly different. Modern processes for both silica manufacturing and bonding chemistry have greatly lessened the differences between phases, making this practice obsolete in most cases. Plots of log k for a given set of test solutes on one column versus log k of the same analytes run under identical conditions on a second column are highly effective at illustrating selectivity similarities or differences (3,4). The selectivity difference due to the change in column chemistry is assessed by the degree of scatter in such a plot. A high degree of scatter means that the analytes react in different ways to the column change and thus show different chromatographic spacing or selectivity. Figure 1 shows such a plot comparing two modern C18 columns. Even in a case where a C18 column built with fully porous particles is compared to a C18 phase constructed on a superficially porous particle, little scatter is observed. The reason for this observation is that both phases exhibit the same fundamental chemistry and thus provide very similar interactions. To change selectivity, different sets of interactions are needed. Method development analysts may alter dominant interactions with a given stationary phase chemistry through changes in parameters such as percent organic, organic type, and pH. However, an often omitted variable, the stationary phase, can be more effective. Figure 2 shows a log k versus log k plot using the same set of analytes as in Figure 1, but here the comparison is between a polar embedded phase and a C18. Note the higher degree of scatter, demonstrating greater selectivity and a higher potential of obtaining different results.
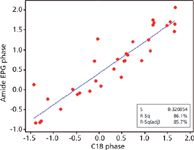
Figure 2: Plot of log k versus log k comparing C18 and amide EPG phases. Conditions same as in Figure 1. C18 phase column: 10 cm à 2.1 mm, 2.7-µm Ascentis Express C18; amide EPG phase column: 10 cm à 2.1 mm, 2.7-µm Ascentis Express RP-Amide.
Embedded Polar Group Phases
Embedded polar group (EPG) stationary phases are characterized as exhibiting an alkyl chain much like their C18 counterparts, but they include a polar group intrinsic to the chain. Figure 3 provides a generalized structure. For the present discussion only the so-called nitrogen-containing EPG phases such as those containing amide, carbamate, or urea functionalities will be discussed. Other phases such as phenyl ether and polar endcapped phases are often labelled as EPG phases; however, these phases tend to provide selectivities that more closely resemble alkyl phases than the nitrogen-containing EPG phases and are beyond the scope of this column instalment (5,6).
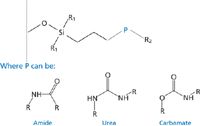
Figure 3: General structures for common nitrogen-containing polar embedded stationary phases.
Since the original EPG phases were first introduced (7), it has been shown that they provide different selectivity compared to classic C18 phases. To predict where and when a particular stationary-phase chemistry will be most effective, it is imperative to have some understanding of the interactions that contribute to different selectivity they provide.

Table 1: LSER comparison of C18 and amide EPG phases.
In an effort to better understand the fundamental interactions that contribute to the differences between C18 and EPG phases, a linear solvation energy relationship (LSER) study was conducted. LSER studies provide a means of interrogating individual contributions of molecular interactions that are collectively manifested as retention and selectivity in chromatographic processes. Refer to reference 8 for an excellent review of LSER methodology. Although classic LSER procedures do not cover all potential interactions (9), the method provides good insight into contributions from hydrogen bonding, lone electron pair interactions, and polarizability. The LSER study presented in this work was conducted by comparing a C18 and an amide EPG phase built on the same silica substrate to emphasize the contribution related only to the surface chemistry. The results presented in Table 1 show that there are significant differences in the polarization and hydrogen bonding terms. The most significant difference between the phases is the ability of the EPG stationary phase to accept a hydrogen bond. Many analytes such as those containing phenolic and aniline groups can readily donate towards a hydrogen bond. If the stationary phase can accept such an interaction, the result should be observable as a difference in selectivity. Figure 4 shows a set of catechol and resorcinol compounds run on both an amide EPG phase and a corresponding C18 phase. Under the stated conditions, the EPG phase provides superior selectivity that is likely because of the additional hydrogen bonding interactions that help differentiate the target analytes. Similar results are indeed observed in many cases where analytes exhibiting hydrogen-bond-donating moieties are present. This observation is consistent with other column classification studies involving nitrogen-containing EPG phases (5,6,10).

Figure 4: Comparison of retention on an embedded polar group phase versus a C18 phase. EPG phase column: 15 cm à 4.6 mm, 5-µm dp Ascentis RP-Amide; C18 phase column: 15 cm à 4.6 mm, 5-µm dp Ascentis C18; mobile phase: 75:25, 20 mM phosphoric acid (pH 2.0 unadjusted)âacetonitrile; column temperature: 30 °C; flow rate: 1.5 mL/min; detection: UV absorbance at 270 nm; injection volume: 25 µL; sample: mixture of catecholamines and resorcinols at 50 µg/mL in 20 mM phosphate buffer (pH 2.0). Peaks: 1 = resorcinol, 2 = catechol, 3 = 2-methyl resorcinol, 4 = 4-methyl catechol, 5 = 2,5-dimethyl resorcinol, 6 = 3-methyl catechol, 7 = 4-nitro catechol.
Classic LSER protocols omit ionic interactions as potential contributing mechanisms. An additional noted difference between alkyl and EPG phases is that the latter tend to attenuate ionic interactions with the silica surface. Hydrophobic bases are notorious for tailing issues when C18 phases are used. The tailing is a result of a small amount of accessible ionized (negatively charged) surface silanols that are present on any silica-based stationary phase. Because hydrophobic bases spend a good deal of residence time within the stationary phase, their probability of interacting with an ionized surface silanol group increases, especially at higher pH values. The presence of a polar group within the alkyl chain effectively masks the ionized silanol groups from the analytes through a number of proposed mechanisms (10,11), often resulting in improved peak shape for such analytes. The effect is especially evident at low pH levels. Figure 5 shows a set of hydrophobic bases chromatographed under the same conditions using an EPG and a C18 phase. As is most often the case, vastly improved peak shape is obtained with the EPG phase. When peak tailing is observed, method development scientists often resort to mobile phase modifiers, such as ion-pairing reagents or surface ionization suppressors (for example, triethylamine) to attenuate the effect. Although these measures can be effective, they often lead to vastly more complicated chromatographic systems that can be difficult to validate, transfer, and troubleshoot. The use of EPG phases most often results in simpler mobile-phase compositions and improved robustness, ruggedness, and reliability.

Figure 5: Comparison of peak shapes for hydrophobic basis on EPG and C18 phases. EPG phase column: 10 cm à 4.6 mm, 3-µm dp Ascentis RP-Amide; C18 phase column: 10 cm à 4.6 mm, 3-µm dp Ascentis C18; mobile-phase A: Water; mobile-phase B: Acetonitrile; mobile-phase C: 0.1% formic acid in water (unadjusted, pH 2.7), 0.1% ammonium formate in water (pH 3.0 with formic acid); gradient: 5â85% B over 8.5 min, hold at 85% B for 1.5 min, %C held constant at 10%; flow rate: 1.5 mL/min; column temperature: 40 °C; detection: UV absorbance at 220 nm; injection volume: 10 µL; sample: 50 µg/mL in 50:50 waterâacetonitrile. Peaks: 1EZ = 4-hydroxytamoxifen (E and Z) isomers, 2 = tamoxifen.
As compared to alkyl phases, EPG phases provide additional polar interactions in the form of (predominantly) hydrogen bond acceptance and less ionic interactions. When an analyte mixture that contains hydrogen bond donors is not well separated on a C18 phase, an EPG phase will often provide the needed selectivity. When hydrophobic bases exhibit tailing on a C18 phase, substitution of an EPG phase often results in improved peak shape. In addition to these two main attributes, EPG phases often provide significant differences in selectivity for polarizable compounds and can be used in 100% aqueous systems (5) without phase dewetting.
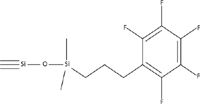
Figure 6: Structure of a pentafluorophenylpropyl (PFP) stationary phase.
Aromatic Stationary Phases
Aromatic stationary phases have also become a popular alternative to alkyl phases (1). In particular, pentafluorophenyl (PFP) phases have emerged as powerful tools in high performance liquid chromatography (HPLC) method development because of their high orthogonality compared to C18 phases. In fact, many newer column lines are introduced with both the classical C18 and some version of a PFP phase. Figure 6 shows the general structure of a PFP stationary phase utilizing a propyl linkage. The strong carbon–fluorine dipoles provide significant dipole-dipole interaction potential. The electronegativity of the fluorine atoms generates a partial negative charge around the outside of the ring as well as a concurrent partial positive charge within the ring system providing the potential for charge transfer interactions. The potential for π-π interactions exists with the aromatic ring system as well as ionic interactions with the silica surface because of the increased spacing between bonded ligands relative to a C18 phase. Lastly, dispersive forces are present that may generate classic partitioning. A log k versus log k plot comparing a PFP and C18 phase is shown in Figure 7. The number of different interactions exhibited by the PFP phase is responsible for the high degree of scatter and thus orthogonal selectivity as compared to C18 columns. In short, the PFP phase provides potential for each of the three classes of interactions described earlier and thus orthogonal selectivity when compared to alkyl phases.
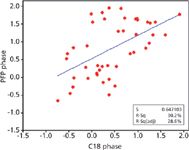
Figure 7: Plot of log k versus log k comparing C18 and PFP phases. Conditions are the same as Figure 1. C18 phase column: 10 cm à 2.1 mm, 2.7-µm Ascentis Express C18; PFP phase column: 10 cm à 2.1 mm, 2.7-µm Ascentis Express F5.
Table 2 shows a comparison of PFP and C18 phases constructed on the same silica substrate using a standard set of chromatographic conditions aimed at retention contributions from hydrophobicity, shape selectivity, hydrogen bonding capacity, and ion-exchange capacity (12). The largest selectivity differences between the phases are in the ionic and shape selectivity terms. These observations are consistent with other reports (12,13). PFP phases exhibit significantly more ion-exchange potential than their corresponding C18 phases (14,15). Figure 8 shows a chromatographic trace of selegiline, amphetamine, and methamphetamine using reversed-phase conditions. Note that the more polar amphetamines are retained longer than the relatively nonpolar selegiline solute. This is in contrast to what is expected in partition chromatography. The amphetamines, being stronger bases, are retaining predominantly via ion-exchange mechanisms, whereas selegiline is retaining predominantly by partition mechanisms. Because ion-exchange and partition interactions are dependent on different variables, the method developer can tailor the mobile phase to provide the desired elution. The power in this is illustrated in Figure 9, where by adjusting both the organic (partitioning) and the buffer concentration (ion exchange) it is possible to elute selegiline before, after, or even between the two amphetamines.

Table 2: Column classification data comparing a C18 and a PFP stationary phase.
A common issue with alkyl phases is that they do not work well for analyte mixtures of highly variable polarity. The method development chemist is often confronted by metabolites or degradation products that are vastly more polar than an active pharmaceutical ingredient (API), for example. Figure 10 shows an example of this. A synthetic impurity and potential degradation product, 2-aminopyridine (2-AMP) is run along with its parent API, piroxicam, on a C18 and a PFP phase. Under isocratic conditions, retention of 2-AMP is not achieved on the C18 without extensive retention of the more hydrophobic parent API. It is possible to retain 2-AMP on the C18 through the addition of an ion-pair reagent; however, a simpler, more robust, and repeatable system can be developed using the intrinsic ion-exchange properties of the PFP phase.
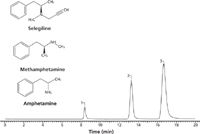
Figure 8: Selegiline and amphetamines retained and separated using a PFP stationary phase. Column: 10 cm à 4.6 mm, 2.7-µm Ascentis Express F5; mobile-phase A: 10 mM ammonium acetate, adjusted to pH 4.0 with acetic acid; mobile-phase B: acetonitrile; mobile-phase composition: 20:80 AâB; flow rate: 1.0 mL/min; column temperature: 35 °C; detection: MS, ESI (+), SIR m/z 136, 150, 188; injection volume: 2 µL; sample: 10 µg/mL each in methanol. Peaks: 1 = selegiline, 2 = amphetamine, 3 = methamphetamine.
Another important property of the aromatic phases is their ability to discriminate based on so-called shape selectivity. Alkyl phases as well as EPG phases possess a high degree of freedom. The rigid structure of the aromatic phases presumably limits the freedom of ligand movement and thus how analytes can physically approach and interact with the stationary phase. An illustration of this is shown in Figure 11. Hydrocortisone and prednisolone differ only in one double bond. Their solubilities and functional groups are essentially identical. The largest difference between the analytes is the three dimensional shape. Where the C18 phase struggles to resolve them, the PFP phase provides good selectivity. Aromatic phases, such as PFP, often provide enhanced selectivity for fused-ring systems (steroids, terpenes, vitamins) (16). Shape selectivity can also be extended to difficult pairs such as positional isomers.

Figure 9: Altering retention and selectivity through manipulation of ion-exchange and percent organic components on a PFP phase. Same conditions as in Figure 8 with the exception of mobile-phase organic component percentage. In each case the analytes in the top, middle, and bottom chromatograms are selegiline, methamphetamine, and amphetamine, respectively.
Aromatic phases such as the PFP chemistry provide enhanced ion-exchange and shape selectivity as compared to their alkyl counterparts. In many cases, the largest differences in a critical pair may be in their ionization constants or shape resulting in incomplete resolution on alkyl phases. PFP and indeed many different aromatic stationary phases often provide the required interactions to discriminate such analytes. The method development scientist often faces a mixture of analytes possessing highly variable polarities. It is often possible to use the aromatic phases and independently adjust both partitioning and ion-exchange mechanisms to tailor the chromatography to a given set of needs. When fused-ring systems and positional isomers fail to separate using traditional alkyl phases, aromatic chemistries generally yield success.
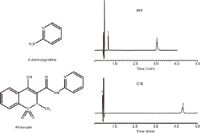
Figure 10: Comparison of retention for compounds of differing polarity on C18 and PFP phases. C18 phase column: 10 cm à 2.1 mm, 2.0-µm dp Ascentis Express C18; PFP phase column: 10 cm à 2.1 mm, 2.0-µm dp Ascentis Express F5; mobile-phase A: 10 mM ammonium formate adjusted to pH 3.0 with formic acid; mobile-phase B: acetonitrile; mobile-phase composition: 75:25 AâB; flow rate: 0.5 mL/min; column temperature: 35 °C; detection: UV absorbance at 250 nm; injection volume: 0.5 µL; sample: 5 µg/mL (2-aminopyridine) and 100 µg/mL (piroxicam) in 90:10 waterâmethanol. Peaks: 1 = 2-aminopyridine, 2 = piroxicam.
Conclusion
Alkyl phases are excellent tools for sample mixtures that differ slightly to moderately in polarity, but often fall short when partition interactions are not enough. EPG phases have been shown to differ from alkyl phases mainly in terms of properties as well as a decrease in ion-exchange capacity because of silanol shielding effects. When analytes that can donate towards a hydrogen bond are present, EPG phases most often provide alternate selectivity and generally provide improved resolution as compared to alkyl phases. Where hydrophobic bases are present and exhibit tailing on C18 phases, the use of low pH on an EPG phase often provides a suitable solution without extensive use of mobile-phase modifiers.
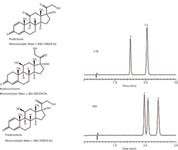
Figure 11: Apparent shape selectivity using C18 and PFP phases. C18 phase column: 10 cm à 2.1 mm, 2.0-µm dp Ascentis Express C18; PFP phase column: 10 cm à 2.1 mm, 2.0-µm dp Ascentis Express F5; mobile phase: 50:50 waterâmethanol; flow rate: 0.5 mL/min; column temperature: 35 °C, detection: UV absorbance at 240 nm; injection volume: 0.5 µL; sample: 50 µg/mL each component in 85:15 waterâmethanol. Peaks: 1 = hydrocortisone, 2 = prednisolone, 3 = prednisone.
Aromatic stationary phases provide additional ion-exchange capacity and shape selectivity as compared to C18 columns. When ion exchange is a desirable mechanism of interaction (analytes differ mostly in pKa values) or when analytes differ mainly in their shape, aromatic phases such as the PFP often provide the necessary selectivity.
The most robust, rugged, and reliable systems are typically the simplest. An understanding of the interactions available from stationary phases other than C18 is paramount to make informed decisions during method development. By choosing the right tools during method development, the simplest, most trouble free chromatographic conditions can be effectively achieved.
Hugh M. Cramer received an associated degree in Business Management from Penn State. He began working at Supelco in 1986 in GC capillary QC. Cramer later supervised the QC area for several years before joining the application area in LC R&D where he has worked for the past 10 years.
Jacinth A.M. McKenzie is an analytical project management (Fellow) at Novartis Pharmaceutical Corporation. Jacinth holds a B.S. and MPhil from the University of the West Indies (Mona, Jamaica). In 1997, she relocated to Gainesville, Florida, USA, and joined the cutting edge separation science research team of Robert Kennedy and received a PhD in analytical chemistry. She spent a short time at a generic pharmaceutical company and quickly determined that day-to-day stability testing and investigations were not attractive. In 2003, she joined the R&D group at Supelco and developed one of the first columns in the Ascentis family. Always seeking a challenge, she moved into the applications group providing technical support to sales, marketing, production groups, and pharmaceutical companies. She is currently an analytical project manager at Novartis Pharmaceutical managing analytical global activities to support INDs and NDAs at various Novartis sites and third party contractors.
Craig R. Aurand is a principal research scientist at Supelco, providing applications development for both chromatographic separations along with sample preparation. Craig's primary role is in support of bioanalytical method development for small molecules, along with R&D product development for sample preparation with an emphasis in mass spectrometry. He is responsible for the development of sample preparation devices for bioanalytical applications at Supelco, with numerous presentations and publications focusing on novel chromatographic separations and sample preparation techniques. His most recent research has focused on the field of microsampling techniques in bioanalysis.
David S. Bell is a manager in pharmaceutical and bioanalytical research at Sigma-Aldrich/Supelco. With a B.S. degree from SUNY Plattsburgh and a PhD in analytical chemistry from The Pennsylvania State University, USA, Dave spent the first decade of his career within the pharmaceutical industry performing analytical method development using various forms of chromatography and electrophoresis. During the past 15 years, working directly in the chromatography industry, Dave has focused his efforts on the design, development, and application of stationary phases for use in HPLC and hyphenated techniques. In his current role at Supelco, Dr. Bell's main focus has been to research, publish, and present on the topic of molecular interactions that contribute to retention and selectivity in an array of chromatographic processes. Direct correspondence to: dave.bell@sial.com
"Column Watch" Editor Ronald E. Majors is an analytical consultant and is a member of LCGC Europe's editorial advisory board. Direct correspondence about this column should be addressed to the editor-in-chief, Alasdair Matheson, at amatheson@advanstar.com
References
(1) R.E. Majors, LCGC Europe 25 (1), 31–39 (2012).
(2) J.W. Dolan, LCGC Europe 21 (5), 258–263 (2008).
(3) D.H. Marchand et al., J. Chromatogr. A 1218 (40), 7110–7129 (2011).
(4) N.S. Wilson, J. Chromatogr. A 961 (2), 217–236 (2002).
(5) M.R. Euerby and P. Petersson, J. Chromatogr. A 1088 (1–2), 1–15 (2005).
(6) N.S. Wilson et al., J. Chromatogr. A 1026 (1–2), 91–100 (2004).
(7) T.L. Ascah and B. Feibush, J. Chromatogr. A 506 (0), 357–369 (1990).
(8) M. Vitha and P.W. Carr, J. Chromatogr. A 1126 (1–2), 143–194 (2006).
(9) M. Reta et al., Anal. Chem. 71 (16), 3484–3496 (1999).
(10) J. Layne, J. Chromatogr. A 957 (2), 149–164 (2002).
(11) J.E. O'Gara et al., Anal. Chem. 71 (15), 2992–2997 (1999).
(12) M.R. Euerby et al., J. Chromatogr. A 1154 (1–2), 138–151 2007).
(13) M. Przybyciel, LCGC Europe 19 (1), 19–28 (2006).
(14) D.S. Bell, H.M. Cramer, and A.D. Jones, J. Chromatogr. A 1095 (1–2), 113–118 (2005).
(15) D.S. Bell and A.D. Jones, J. Chromatogr. A 1073 (1–2), 99–109 (2005).
(16) C.R. Aurand, D.S. Bell, and M. Wright, Bioanalysis 4 (22), 2681–2691 (2012).

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Determining the Serum Proteomic Profile in Migraine Patients with LC–MS
April 17th 2025Researchers used liquid chromatography–mass spectrometry (LC–MS) in their proteomic analysis to compare the serum proteome of migraine patients with healthy controls and to identify differentially expressed proteins as potential migraine biomarkers.










