Rapid Quality Control of Flavours and Fragrances using Fast GC–MS and Multi-MS Library Search Procedures
LCGC Europe
Using narrow-bore column fast GC combined with rapid scanning qMS to analyse flavours and fragrances is described.
This article discusses the use of narrow-bore column fast gas chromatography (GC) combined with a rapid-scanning quadrupole mass spectrometer (qMS) to analyse flavour and fragrance (F&F) samples. Sample and peak identification was achieved with the support of two MS libraries. The first library contained specific pure spectra, relative to entire F&F volatile fractions. Each F&F spectrum was attained by fast gas chromatography mass spectrometry (GC–MS) analysis and can be considered, in terms of concept, in the same way as a direct ion-source injection of the sample. Once the F&F was correctly identified the second MS library was applied: each peak was assigned on the basis of spectral similarity and linear-retention-index data. The narrow-bore column fast GC–qMS method developed was subjected to validation.
The use of gas chromatography mass spectrometry (GC–MS) is common in the field of flavour and fragrance (F&F) analysis. GC–MS is a highly valuable tool and can be regarded as a bidimensional technology: the separation dimension delivers sample fractions to the MS, which subjects the fractions to fragmentation [usually through electron ionization (EI)], and then separates the generated ions on a m/z basis.
The analysis of an unknown F&F sample is a challenging task, requiring basically two identification levels: (1) the sample must be correctly identified at mixture level; (2) the individual sample components must be correctly identified at the molecular level. Considering level 1, one way to identify an unknown flavour or fragrance could be to introduce the sample directly into the MS ion source and directly identify the attained spectrum (if you are an experienced MS analyst) or carry out a library search process (if you have an EI-MS library with F&F spectra). Depending on the degree of volatility, samples can be introduced into the ion source by using introduction systems such as a direct insertion probe, a direct exposure probe or a heated reservoir.1 Once a F&F has been identified through EI-MS, then one must pass to the second identification stage; generally achieved through GC–EI-MS. In contemporary F&F analysis, stationary phases of various polarities are used, while identification is usually carried out by exploiting MS spectra and calculating linear retention indices (LRI).2 In previous research, a novel double-filter MS library has been developed and applied to the analysis of F&F volatiles.3,4

KEY POINTS
The double filter worked in the following manner: at the end of the analysis the GC–MS software automatically calculated LRI values relative to each peak. After — and during — the MS library search process the same software deleted matches with a reference LRI, with respect to that of the unknown, outside a pre-defined LRI range (e.g., ±5 units). The latter is dependent on the type of GC stationary phase used: apolar phases are characterized by lower LRI variations, compared with polar ones. The LRI values contained in the library were derived from GC–MS analysis carried out on a 30 m × 0.25 mm i.d., 0.25 µm film thickness (5% diphenyl + 95% polydimethylsiloxane) column.
The present research is focused on four main objectives: (1) the development and validation of a fast GC–EI-MS method, using an apolar narrow-bore 10 m × 0.1 mm i.d. capillary, for the analysis of F&F samples; (2) the evaluation of the effectiveness of the previously reported double-filter MS library, under the fast GC–EI-MS conditions developed. Specific attention was devoted to the definition of an LRI window for fast GC–EI-MS analysis, using a silphenylene polymer (equivalent in polarity to a 5% diphenyl + 95% polydimethylsiloxane phase) column; (3) the development of a GC–EI-MS F&F library, defined as EI-MS F&F, for the identification of unknown samples at mixture level. The library was constructed by subjecting genuine F&F samples to the fast GC–EI-MS method developed and then deriving a single averaged spectrum comprising all the peaks in the retention time range. The F&F spectrum attained can be considered as a sample fingerprint and is altogether comparable, in terms of concept, to direct EI-MS analysis; (4) evaluation of the fast GC–EI-MS method combined with the two MS libraries for the rapid quality control of F&F samples.
Samples and Sample Preparation
The genuine essential oil samples were kindly provided by Citrus Vita (Pace del Mela, Messina, Italy), Simone Gatto (San Pier Niceto, Messina, Italy) and Dr Pappas (Essential Oil University, New Albany, USA). All samples were stored at +4°C. Prior to analysis, each essential oil was diluted 1:10 (v/v) in n-hexane.
The C7–C30 alkanes and pure standard compounds listed in Table 1 were supplied by Sigma-Aldrich (Milan, Italy). A C7–C30 solution, at the 100 ppm level, was prepared in
n-hexane.
GC–MS Operational Conditions
All GC–MS applications were carried out on a Shimadzu GCMS-QP2010 Plus instrument (Kyoto, Japan), equipped with an AOC-20s auto sampler and an AOC-20i auto injector.
Fast GC–MS: The column used was an SLB-5ms 10 m × 0.10 mm i.d. × 0.10 µm df (silphenylene polymer) and was kindly provided by Supelco (Milan, Italy). The temperature programme used was from 50–250 °C at 10 °C/min; the carrier gas (helium) was delivered at an initial pressure of 343.2 kPa (constant linear velocity mode: 50 cm/s). Injected sample volume: 0.5 µL; split ratio: 400:1.
Conventional GC–MS: The column used was an SLB-5ms 30 m × 0.25 mm i.d. × 0.25 ×m df, and was kindly provided by Supelco. The temperature programme used was from 50–250 °C at 3 °C/min; the carrier gas (helium) was delivered at an initial pressure of 32.0 kPa (constant linear velocity mode: 30.8 cm/s). Injected sample volume: 1 µL; split ratio: 100:1.
MS parameters: The sample was analysed in the full scan mode with a scan speed of 3333 amu/sec, a mass range of 40–350 m/z and a sampling frequency of 10 spectra/s, under fast GC–MS conditions; a scan speed of 700 amu/sec, a mass range of 40–350 m/z and a sampling frequency of 2 spectra/s, were applied under conventional GC–MS conditions; interface and ion source temperatures were 250 °C and 200 °C, respectively.
MS ionization mode: Electron ionization (EI); detector voltage: 1.0 kV. Data were collected by the GCMSsolution software (Shimadzu). The MS libraries used for spectral matching were the EI-MS F&F and the FFSNC 1.4 (Chromaleont, Messina, Italy).
Results and Discussion
In the field of vacuum-outlet fast GC, it is well-known that the use of short mega-bore capillaries is an attractive option for the rapid analysis of low-complexity samples; in fact, low-pressure conditions extend across the entire capillary length, analyte-gas diffusion coefficients are increased and hence, optimal linear velocities are higher. Some very rapid fast GC–MS separations have been reported in the literature.5–7 However, the use of megabore columns in fast GC–MS experiments is not advisable in the analysis of medium or high-complexity matrices, such as F&F ones. In that situation, it is more convenient to employ a narrow-bore column, offering a much higher plate number. In capillary GC, a reduction in internal diameter enables a substantial increase in column efficiency: for columns with a high phase ratio, the minimum H value approaches the column internal diameter. Consequently, a 10 m × 0.10 mm i.d. × 0.10 µm narrow-bore capillary generates the same theoretical plate number (100000), with respect to a 25 m × 0.25 mm i.d. × 0.25 µm column, if both are operated under optimal conditions. Furthermore, the ascending portion of the Golay curve for a narrow-bore capillary raises very gradually and, hence, higherthanoptimal gas velocities can be applied with little loss of column efficiency. Finally, the optimal linear velocity for a given column increases with an i.d. reduction. Hence, short narrow-bore columns enable rapid GC analysis and can provide results altogether comparable to conventional GC experiments, in terms of resolution.8–13
The GC method described here is a relatively fast one: an optimal constant He velocity of 50 cm/s was applied, with a linear temperature gradient of 10 °C/min. Initially, a C7–C30 alkane mixture was subjected to rapid analysis, for LRI calculation. After, a genuine lemon essential oil was analysed: a TIC GC–qMS chromatogram, with a run time of less than 9 min (around 4–5 times faster than a conventional GC–MS experiment), is reported in Figure 1.
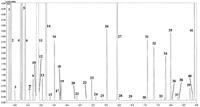
Figure 1: TIC fast GCâqMS chromatogram of lemon essential oil. Peak identity: 1) α-thujene; 2) α-pinene; 3) camphene; 4) sabinene; 5) β-pinene; 6) myrcene; 7) octanal; 8) α-phellandrene; 9) α-terpinene; 10) p-cymene; 11) limonene; 12) β-ocimene (Z); 13) β-ocimene (E); 14) γ-terpinene; 15) cis-sabinene hydrate; 16) terpinolene; 17) linalool; 18) trans-sabinene hydrate; 19) nonanal; 20) camphor; 21) citronellal; 22) terpinen-4-ol; 23) α-terpineol; 24) decanal; 25) nerol; 26) neral; 27) geranial; 28) perillaldehyde; 29) undecanal; 30) citronellyl acetate; 31) neryl acetate; 32) geranyl acetate; 33) α-bergamotene cis; 34) caryophyllene (E); 35) α-bergamotene trans; 36) β-farnesene (Z); 37) β-farnesene (E); 38) β-santalene; 39) bicyclogermacrene; 40) α-bisabolene; 41) β-bisabolene.
Band widths were rather wide for a 6σ) for α-thujene (tR = 1.778 min), terpinolene (tR = 3.516 min) and geranyl acetate (tR = 7.341 min) were 1.8 s, 2.4 s and 2.7 s, respectively. The 10 Hz sampling frequency of the rapid-scanning qMS, generated a sufficient number of spectra per peak for proper peak re-construction. Moreover, the spectra profiles were consistent over each peak and, hence, peak skewing was not observed. Forty-one well-known lemon oil volatiles were identified (see Figure 1) by using the double-filter FFNSC MS library; which was, as previously mentioned, constructed through conventional GC–qMS analysis. The first filter eliminated possible library matches with a spectral similarity < 90%. For the second filter, an initial wide LRI window was applied (±20 units) to evaluate the differences between the experimental (automatically calculated through the GC–qMS software) and library values. In conventional GC–qMS analysis, using the same narrow-bore column stationary phase, a ±5 LRI unit window is usually applied. In the fast GC–qMS analyses, it was observed that a ±5 unit window would have been sufficient for all the identified compounds except for octyl acetate (+9 units), caryophyllene (E) (–9 units), and bicyclogermacrene (–6 units). Consequently, it was concluded that a ±10 LRI unit range would be suited for the analysis of six additional essential oil types, namely mandarin (green, yellow, red), bergamot, sweet and bitter orange. Each citrus oil was subjected to five fast GC–MS experiments, on the same day and on five consecutive days; the C7–C30 alkane mixture was analysed prior to each application. The final results attained indicated that the ±10 LRI unit range was suitable for the fast GC–qMS method; however, a widened range of ±15 LRI units would be more advisable because a few analytes were characterized by a 10 LRI unit difference, with respect to the library value. On the basis of the authors' experiences, the differences between conventional and fast GC LRI values tend to be more substiantial when the difference between total analyses times increases. Hence, the ±15 LRI window used is valid for the fast GC–qMS method herein developed; a wider LRI range (e.g., ±20, ±25, etc.) would be necessary for "faster" GC–qMS experiments (i.e., very-fast GC).
The fast GC–qMS method was subjected to validation (lemon oil was used) in terms of intra- and inter-day (n = 10) retention time and peak % area precision. CV% values for 10 lemon oil compounds (peaks 1, 3, 5, 9, 13, 14, 16, 23, 27 and 31) were never over 0.2% (intra-day) and 0.3% (inter-day) if tR precision is considered, and always lower than 9.8% (intra-day) and 10.6% (inter-day) for peak % area repeatibility. Intra- and inter-day LRI values were also very stable: a C7–C30 alkane series was analysed prior to the lemon essential oil; the process was carried out five times on the same day and on five separate days. No LRI CV% value, relative to the same 10 peaks, was found to be over 0.2%. Finally, the minimum concentration value, at which the 10 compounds (analysed as pure standards) were identified with a minimum MS similarity of 90% (n = 10), was 10 ppm, apart from γ-terpinene which was identified with a 90% similarity at the 5 ppm level.
At this point, the same fast GC–qMS method was used for the construction of a further MS library, defined as EIMS F&F. To date, approximately 200 genuine essential oils have been subjected to fast GC–qMS and a single spectrum, derived from each chromatogram, is appended in the library. The procedure for the construction of the EIMS F&F library is based on the averaging of the spectra relative to all the chromatogram peaks, from the first to last, not including the solvent, without subtracting an average spectrum relative to the baseline. The baseline spectrum was not subtracted because it would be highly difficult to obtain an average baseline spectrum relative to the entire chromatogram elution range. In terms of concept, each EIMS F&F spectrum can be considered in the same way as a direct MS injection, even though no direct comparison was made between the two methodologies. It is obvious that the EI-MS F&F library can accurately identify an unknown sample but not its constituents (the ability of the approach to deal with natural compositional variation will be addressed below). The essential oils analysed were all diluted 1:10 (v/v) in n-hexane, injected in a 0.5 µL volume, with a 400:1 split ratio. A 90% similarity filter is applied using this novel library, whereas the LRI filter is obviously not applicable. The EI-MS F&F library was tested by analysing 10 blind samples provided by essential oil companies, all correctly identified by the EI-MS F&F library, with MS % similarities of at least 96%. A single example is shown in Figure 2: the averaged spectrum was subjected to library matching, and the unknown oil was identified as geranium oil (Pelargonium roseum) with a single 98% match.
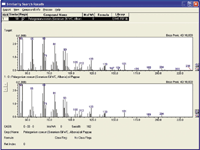
Figure 2: EI-MS F&F library result: the experimental spectrum is the upper one.
At this point, the fast GC–qMS methodology, in combination with the FFNSC and EI-MS F&F libraries, was evaluated. Another of the ten blind samples was subjected to recognition; the fast GC–qMS chromatogram relative to the unknown sample is illustrated in Figure 3(a).

Figure 3: a) TIC fast GCâqMS chromatogram relative to an unknown F&F sample; b) EI-MS F&F library result.
The EI-MS F&F search provided an excellent 100% spectral similarity for green mandarin oil [Figure 3(b)]. However, as can be observed, various green mandarin oils, produced in different harvesting periods (during autumn 2008), were present in the "hit" list. In this specific case, the correct match was the first in the list (harvesting period: 30 October–12 November 2009), although in several other cases, the MS library procedure could not distinguish between green oils produced in different periods. Moreover, yellow and red mandarin essential oils were also present in the "hit" list with excellent similarities and, hence, it was concluded that the EI-MS F&F library cannot reliably distinguish between green, yellow and red mandarin oils. However, careful evaluation of target constituents can be of great help in the differentiation of mandarin oil samples. For example, it is known that the limonene content increases during the production season, ranging from an average value of 68% in October to 74% in February. Furthermore, some monoterpene hydrocarbons (γ-terpinene, β-pinene, and α-pinene), the sesquiterpene hydrocarbons and the oxygenated compounds decrease during the production season.14
The green mandarin oil chromatogram was then subjected to peak-to-peak recognition, with a total number of 34 compounds identified (Table 1).
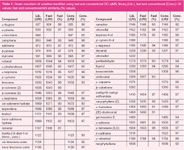
Table 1: Green mandarin oil volatiles identified using fast and conventional GCâqMS; library (Lib.), fast and conventional (Conv.) LRI values; fast and conventional MS similarity (%) values.
With regards to the library LRI values (a ±15 unit window was used), maximum differences of + and – 10 LRI units were observed for limonene and α-selinene, respectively. Moreover, an average deviation of 3.3 LRI units (absolute value), with respect to the library LRIs, was observed. Considering the spectral quality, 14 mandarin constituents (~41%) were identified with an MS similarity value of at least 95%. The peak-to-peak identification results were then compared with those derived from a conventional optimized GC–qMS experiment on green mandarin oil. The total analysis time was more than five times higher (~50 min) and, as expected, the higher resolving power of a 30 m × 0.25 mm i.d. capillary (circa 120000 N) enabled the separation and identification of a higher number of compounds, namely 41 (Table 1). With regards to LRI values (a ±5 unit window was applied), maximum differences of 5 LRI units were observed for various terpenes, suggesting that a wider window would be advisable. The average LRI deviation, with respect to the library LRIs, was improved, with a calculated value of 1.6. Finally, spectral quality was very good, with 31 volatiles (~76%) identified with an MS similarity value of minimum 95%. Again, the better similarity values attained in the conventional application are certainly due to the increased column efficiency. Finally, an averaged spectrum was derived from the conventional GC–qMS chromatogram and was subjected to an EI-MS F&F library search: 94% and 93% similarity matches were attained for yellow and green mandarin oils, respectively. Further EI-MS F&F library searches were carried out on other conventional F&F GC–qMS chromatograms and it was concluded that, although the EI-MS F&F library can be used in conventional analysis, the best results were certainly attained with the fast GC–qMS methodology.
Conclusions
The results attained in this investigation can certainly be considered as a positive contribution to the F&F field; in fact, the step-wise process proposed enables a rapid identification and evaluation of unknown F&F matrices. The newly-developed EI-MS F&F library, which is currently under continuous growth, is certainly a promising and innovative tool. Moreover, the dualfilter MS library, initially developed for conventional GC–qMS analysis, has been proven suitable for the validated, rapid GC–qMS approach. The use of both libraries, combined with the fast method, can provide a reliable analytical result in less than an hour, comprising sample preparation, alkane mixture and F&F fast GC–qMS analysis and data analysis.
Future research will be devoted to the development of other dedicated MS libraries, to be used in the field of monodimensional and bidimensional GC–qMS analysis.
Acknowledgments
The Project was funded by the Italian Ministry for the University and Research (MUR) with a PNR 2005-2007 Project n. RBIP06SXMR "Sviluppo di metodologie innovative per l'analisi di prodotti agroalimentari".
The Authors gratefully acknowledge Shimadzu Corporation and Supelco Corporation for the continuous support.
Maria Scandinaro is attending the final year in the Ph.D. course entitled "Food Chemistry and Safety" at the University of Messina and is engaged in the development of MS databases for food analysis.
Peter Q. Tranchida is assistant professor of food chemistry at the University of Messina. His main interests are the analysis of food constituents using fast gas chromatography, as well as classical multidimensional and comprehensive chromatographic techniques.
Rosaria Costa is principal scientist of Chromaleont, a spin-off society located at the University of Messina. She received a Ph.D. in "Production and Safety of Food of Animal Origin" from the University of Sassari, Italy.
Paola Dugo is associate professor of food chemistry at the University of Messina and at the "Campus Biomedico" in Rome. Her research interests include the development of comprehensive chromatography and high throughput separation methods for the study of food components.
Giovanni Dugo is a full professor of food chemistry at the University of Messina. His main research interests are focused on the development of innovative chromatographic techniques and mass spectrometric methods for the study of complex food samples.
Luigi Mondello is a full professor of analytical chemistry at the University of Messina and at the "Campus Biomedico" in Rome. His main research interests are focused on comprehensive chromatographic techniques, fast separation methods and mass spectrometric detection.
References
1. J.H. Gross, in Mass Spectrometry: A Textbook, (SpringerVerlag, Berlin-Heidelberg 2004), pp.206–213
2. P.J. Marriott, R. Shellie and C. Cornwell, J. Chromatogr. A, 936(1-2),1–22 (2001).
3. L. Mondello et al., J. Sep. Sci., 28(9–10), 1101–1109 (2005).
4. L. Mondello et al., J. Chromatogr. A, 1067(1–2), 235–243 (2005).
5. M.M. van Deursen et al., J. Microcol. Sep., 12(12), 613–622 (2000).
6. K. Maštovska, S.J. Lehotay and J. Hajšlova, J. Chromatogr. A, 926(2), 291–308 (2001).
7. S.D.H. Poynter and R.A. Shellie, J. Chromatogr. A,1200(1), 8–33 (2008).
8. P. Donato et al., J. Sep. Sci., 30(4), 508–526 (2007).
9. P.Q. Tranchida et al., J. Sep. Sci., 31(14), 2634–2639 (2008).
10. V.R. Reid and R.E. Synovec, Talanta, 76(4), 703–717 (2008).
11. L. Mondello et al., J. Chromatogr. A, 1035(2), 237–247 (2004).
12. L. Mondello et al., J. Agric. Food Chem., 51(19), 5602–5606 (2003).
13. L. Mondello et al., J. Pharm. Bio. Anal., 41(5), 1566–1570 (2006).
14. G. Dugo et al., in Citrus, G Dugo and A. Di Giacoma, Eds (Taylor and Francis, London, 2002), p. 247.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.













