Rapid Extraction and Analysis of PPCPs From Sediments by QuEChERS and Liquid Chromatography Tandem Mass Spectrometry
Pharmaceutical and personal care products (PPCPs) are routinely detected in a variety of aquatic environments and these compounds encompass a wide range of chemical and physical properties that contribute to their combined analytical screening challenges. Further examination of their accumulation throughout environmental samples that contain solids, including sediments, adds additional levels of analytical difficulties in their effective extraction and new sample matrix interferences. QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) has become a very popular extraction and cleanup technique for the analysis of multiresidue pesticides in agricultural and food samples. Applying the same principles to overcome similar challenges in PPCP analysis in solid environmental samples is demonstrated in this article.
kenkistler1/stock.adobe.com

Pharmaceutical and personal care products (PPCPs) are routinely detected in a variety of aquatic environments and these compounds encompass a wide range of chemical and physical properties that contribute to their combined analytical screening challenges. Further examination of their accumulation throughout environmental samples that contain solids, including sediments, adds additional levels of analytical difficulties in their effective extraction and new sample matrix interferences. QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) has become a very popular extraction and cleanup technique for the analysis of multiresidue pesticides in agricultural and food samples. Applying the same principles to overcome similar challenges in PPCP analysis in solid environmental samples is demonstrated in this article.
Among the myriad of contaminants of emerging concern (CECs) that are expanding and persisting throughout the environment are a broad group of pharmaceuticals and personal care products (PPCPs) that include steroid hormones, industrial chemicals, and pesticides. These will reach the environment through routine human usage and disposal in wastewater where they can settle within diverse pockets of moist sediments.
A common technique for extracting a wide range of multiresidue pesticides out of both wet and dry solid agricultural and food samples is QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe). This technique is essentially a facilitated solvent extraction with salts into acetonitrile followed by dispersive solidâphase extraction (dSPE). When analyzing hundreds of multiresidue pesticides with diverse chemical and physical properties, it can be difficult, if not impossible, to provide effective sample cleanup through traditional retentive solidâphase extraction. As such, instead of targeting retention of analytes of interest, dSPE is applied to target removal of sample matrix components. Primary secondary amine (PSA) sorbents are routinely used to target and remove excess organic acids, small fatty acids, sugars, and anthocyanins. Endcapped C18 (C18E) sorbents are routinely used to target and remove excess fats, sterols, and other nonpolar interferences. Graphitized carbon black (GCB) sorbents are routinely used to target and remove pigments. These are the routine dSPE sorbents used in a traditional QuEChERS cleanup, and there are also customized dSPE sorbents designed to remove other specific matrix interferences without losing recovery of some analytes of interest.
Because parallel challenges exist for PPCPs in sediment analysis (effective extraction from solid matrices and many analytes with diverse chemical properties), a modified QuEChERS approach can be an elegant solution applied to the same underlying chemistry principles.
Methods
Sample Preparation-Modified QuEChERS Extraction: Two grams of dried sediment were weighed into a 50âmL polypropylene vessel and spiked with internal standard. Two preparations are required for each sample to cover the positive ion-mode and negative ion-mode analyses. The same sample preparation applies to each, and in this article the positive ion-mode case is covered. Sand was used for the method blanks, and sediment and sand spiked with internal standards and recovery standards were used for the control sample.
A 10-mL measure of deionized water was added and vortexed, followed by 10 mL of 1% acetic acid in acetonitrile, and the subsequent slurry was vortexed.
Sodium Acetate and Magnesium Sulfate Extraction: QuEChERS salts were added and the slurry vortexed, and then the sample was centrifuged for 5 min at 4000 rpm. Samples were then placed in a rack at -20 °C for 2 h to facilitate the extraction of the supernatant.
An 8-mL measure of the acidified acetonitrile supernatant was then transferred to a tube containing QuEChERS dSPE PSA sorbent (Phenomenex). For negative ionâmode analysis, the supernatant would be cleaned up with PSA and C18 dSPE sorbent.
Tubes were then centrifuged for 10 min at 3000 rpm, and the supernatant decanted and filtered through a 0.2âµm syringe filtered before drying under a gentle stream of nitrogen at or below 35 °C.
A 50-µL measure of acetone was then added to the dried sample and vortexed to dissolve any residue; 950 µL of 50% methanol in water solution was then added ready to transfer to an autosampler vial for analysis.
The liquid chromatography tandem mass spectrometry (LC–MS/MS) analysis was performed on a 50 × 2.1 mm, 2.6-µm Kinetex core–shell C18 column (Phenomenex) with a formic acid in water and methanol gradient in 8 min on a Triple Quad 4500 (Sciex) (Figure 1).
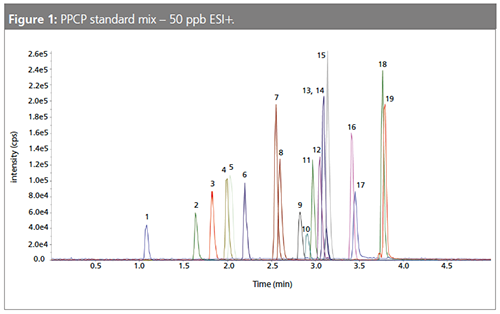
Results and Discussion
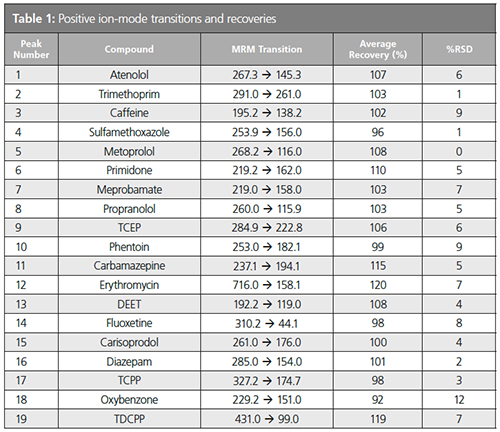
Average spike recoveries were within 80–120%, and precision was below 15% RSD (Table 1).
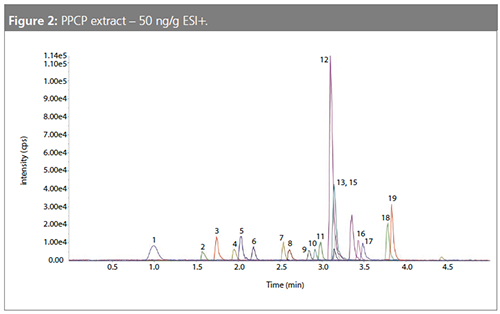
The modified QuEChERS method proved to be a very simple and effective method for the determination of PPCPs in sediments. Sample throughput was improved significantly by reducing the average sample processing time to less than 4 h compared to upwards of 12 h for a typical Soxhlet extraction.
Ion suppression and enhancement challenges were also reduced by removing sediment interferences in the dSPE step of the sample preparation (Figure 2).
Upon sample dry-down, sometimes a brown residue persisted that could hold onto some of the analytes and internal standards and decrease their recovery. Adding a small amount of acetone to initiate the sample reconstitution helped to dissolve this residue and improve recoveries.
Conclusion
PPCPs are detected in many different aquatic environments and aside from analyzing the water source directly, sediments must also be analyzed to understand the fate of these compounds. The outlined modified QuEChERS extraction protocol effectively recovers these diverse range of compounds from the solid and semisolid sediments and eliminates matrix interferences that can disrupt accurate LC–MS/MS analysis.
Acknowledgements
Special thanks to the Sanitation Districts of Los Angeles County – San Jose Creek Water Quality Laboratory for their contributions.
Scott Krepich is the Global Industry Manager for Food and Environmental Testing at Phenomenex, with over 20 years of chromatography experience. After studying biochemistry at the University of Illinois at UrbanaâChampaign, USA, he worked as an HPLC and GC method development scientist at American Pharmaceutical Partners in Melrose Park, Illinois, USA. Scott has been with Phenomenex for 13 years and has given hundreds of presentations and onsite demonstrations on sample preparation, chromatography, and total workflow solutions throughout the globe.
E-mail:ScottK@Phenomenex.comWebsite: www.Phenomenex.com

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











