Enhanced Detection of Oxygenated Polycyclic Aromatic Hydrocarbons Using Atmospheric-Pressure Chemical Ionization-Mass Spectrometry
The Column
Polycyclic aromatic hydrocarbons (PAHs) and their oxygenated derivatives (oxy-PAHs) are highly toxic carcinogens that present a significant hazard to human health. To fully understand the risks associated with exposure to PAHs, robust analytical methods for their detection are required. Mass spectrometry coupled with ultrahigh-performance liquid chromatography (UHPLC–MS) has proven to be a powerful technique for the analysis of these compounds. This article looks at the benefits of using atmospheric-pressure chemical ionization (APCI) in the place of traditional electrospray ionization (ESI) for the detection of oxy-PAHs.
Bede/stock.adobe.com

Polycyclic aromatic hydrocarbons (PAHs) are a diverse family of environmental pollutants that are widely recognized as highly toxic and carcinogenic to humans (1). Comprised of multiple fused aromatic rings, these compounds are produced by the incomplete combustion of carbonâbased materials, predominantly as a result of human activity, such as the burning of fuel for cooking and heating, vehicle emissions, and power generation, but also from natural phenomena, such as wildfires. PAHs can undergo further reactions in both the gas and condensed phase to produce oxygenated derivatives (oxy-PAHs), which have similar harmful health effects (1).
Individuals may be exposed to PAHs and oxy-PAHs in different ways, with food being a major source (2,3). PAHs can enter the food chain by deposition and transfer from air or soil, or through specific food preparation practices. For example, foods that are grilled, roasted, or smoked during preparation can contain higher levels of PAHs (3). Given the serious health concerns associated with PAHs, and the high risk of human exposure by inhalation and ingestion, the analysis of these compounds in a wide range of materials has been the focus of several environmental and nutritional studies (2–5).
Gas chromatography–mass spectrometry (GC–MS) has proven to be a capable analytical technique for the analysis of PAHs (1,4,5). However, for more polar, functionalized variants, such as oxyâPAHs, reduced volatility and greater potential for adsorption mean these substances often cannot be detected with sufficient sensitivity using GC–MS. While derivatization can sometimes be used to overcome these analytical challenges (5), this process can be time-consuming and derivatization reactions may not always proceed to completion.
Alternative techniques based on high performance liquid chromatography (HPLC) coupled with either optical or MS detection offer a faster and more convenient approach to oxyâPAH analysis (1,6). While electrospray ionization (ESI) is generally the preferred ionization technique in most LC–MS applications, studies have shown that atmosphericâpressure chemical ionization (APCI) offers improved performance when it comes to oxy-PAH analysis (1,7). This article compares APCIâMS and ESI-MS detection for the analysis of six oxy-PAH standards using ultrahighâperformance liquid chromatography (UHPLC) and otherwise identical conditions.
Experimental
Standard Preparation: Standard stock solutions of 2-naphthoic acid, 2-naphthol, benzanthrone, 1-hydroxypyrene, 1,4-anthraquinone, and 1-tetralone were prepared in methanol at a concentration of 1 mg/mL. A working solution for injection into the UHPLC–MS system was prepared by mixing the stock solutions and diluting with methanol to give a final concentration of 10 µg/mL for each analyte.
Analytical Conditions: A Vanquish Flex Binary UHPLC system (Thermo Fisher Scientific) equipped with an ISQ EM single quadrupole mass spectrometer (Thermo Fisher Scientific)
was used for the analysis. Chromatographic separation was achieved using a 150 × 2.1 mm, 3-µm Hypersil Green PAH column (Thermo Fisher Scientific), using a water–methanol gradient method and sample injection volume of 1 µL.
Data Analysis: Chromeleon 7.2.9 Chromatography Data System software (Thermo Fisher Scientific) was employed for data acquisition and processing.
Detection of Oxy-PAH Standards Using APCI-MS and ESIâMS
Baseline separation for all six oxy-PAH standards was achieved (Figure 1), as well as small amounts of impurities visible in the UV chromatogram, which may have resulted from synthesis of the standard compounds.
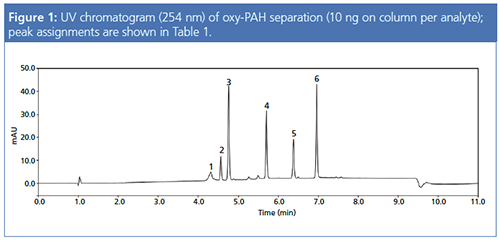
Switching from ESI to APCI detection could be achieved by changing the source probe and turning the APCI needle to the appropriate position. The molecular masses and most abundant mass-to-charge ratio (m/z) values for the six oxy-PAHs detected using the various experimental conditions are shown in Table 1. As a result of the different ionization mechanisms associated with each ionization mode, in some cases the ions generated were not the same.
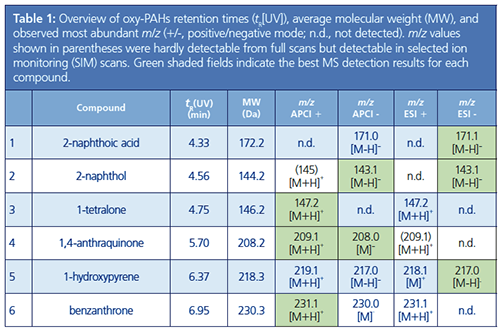
For both ESI and APCI, when operating in positive mode, most ions formed as the protonated molecular ion [M+H]+. In negative mode, formation of the deprotonated molecular ion [M-H]-
was favoured. Notable exceptions were 1-hydroxypyrene, 1,4-anthraquinone, and benzanthrone. 1-Hydroxypyrene was detected as the molecular ion [M]ï« in positive ESI mode. Although 1,4-anthraquinone and benzanthrone were detected as the molecular ion [M]- in negative APCI mode, these were not detected in negative ESI mode.
This difference is due to a process known as electron capture negative ionization, which is observed when using APCI for molecules that lack an abstractable hydrogen.
1-Hydroxypyrene was detected under all four experimental conditions. 2-Napthoic acid, 2-napthol, and 1-tetralone were detected using both APCI and ESI, although in just one polarity in full scan mode. Using a selected ion monitoring (SIM) scan of the [M+H]+ ion, 1-napthol was also detected in positive APCI mode. The [M+H]+ ion of benzanthrone was easily detected in both positive ionization modes. However, while APCI generated the [M]- ion in negative mode, its detection was not possible in negative ESI mode. 1,4-Anthraquinone provided good signals in positive and negative polarity modes using APCI, but was not observed in either of the full scans using ESI. Only by performing a SIM scan could the [M+H]+ be detected under ESI conditions, albeit as a weak signal.
As well as the oxy-PAH molecular ions, additional species attributed to adducts were observed in the ESI spectra. In negative mode, a signal at m/z 365.0 was detected for 2-naphthoic acid, while in positive mode, signals at m/z 247.1 and 301.2 were detected for 1-hydroxypyrene and at m/z 253.1 and 285.1 for benzanthrone. By contrast, when using APCI, just one adduct was observed at m/z 179.1 for 1-tetralone.
Detection of Oxy-PAHs Using APCIâMS
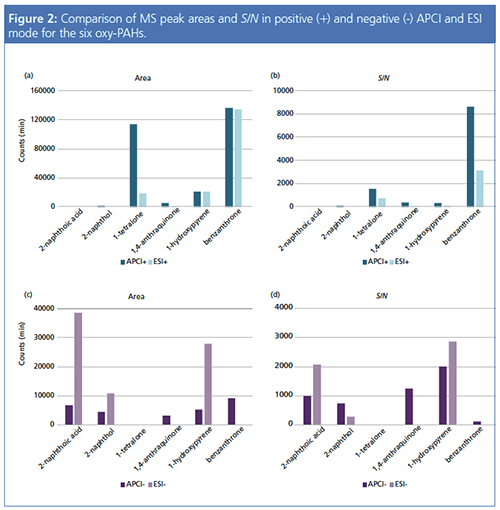
The MS peak areas and signal-to-noise ratio (S/N) obtained for the most abundant m/z signals under all four experimental conditions are compared in Figure 2. Figures 2(a) and 2(b) reveal that the signal responses obtained using APCI in positive mode were comparable or higher than with ESI, while the S/N achieved for each of the detected compounds were better using APCI. Figures 2(c) and 2(d) show that the peak areas for the three compounds detected using ESI negative mode were higher than those recorded using APCI negative mode. However, the S/N is only higher for two of these analytes (2-napthoic acid and 1-hydroxypyrene). Importantly, APCI operating in negative polarity mode enables the detection of two additional analytes (1,4-anthraquinone and benzanthrone).
Conclusion
Although conventional ESI showed higher detection sensitivity for some analytes when operating in negative polarity mode, APCI enabled convenient detection of all six oxyâPAHs studied. This compares to just one of the standards being barely detectable when using ESI mode. Taken together, these results demonstrate that APCI-MS offers several benefits over ESIâMS, and may be considered the preferred ionization mode for UHPLC–MS analysis of oxy-PAHs.
References
- R.E. Cochran, I.P. Smoliakova, and A. Kubatova, Int. J. Mass Spectrom. 397–398, 6–17 (2016).
- L. Singh and T. Agarwal, Trends Food Sci. Technol. 79, 160–170 (2018).
- Z. Zelinkova and T. Wenzl, Polycycl. Aromat. Compd.35, 248–284 (2015).
- C. Walgraeve et al., Anal. Bioanal. Chem.409, 335–347 (2017).
- S. Lundstedt, Anal. Chem.57, 83–92 (2014).
- S. Kumar, S. Negi, and P. Maiti, Environ. Sci. Pollut. Res. 24, 25810–25827 (2017).
- S. Grosse and T. Letzel, J. Chrom. A1139, 75–83 (2007).
Maria Grübner has worked as an application chemist in HPLC at Thermo Fisher Scientific since 2017. Her degree in food chemistry and her time as the Chair for Food Chemistry and Sensory Sciences of the Technical University Munich means that she is very familiar with various analytical techniques with a main focus on liquid chromatography and mass spectrometry.
Frank Steiner manages HPLC application development and coordinates HPLCâbased scientific collaborations in Thermo Fisher Scientific. Frank joined Dionex Corporation, now a part of
Thermo Fisher Scientific, in 2005 and had been manager in different HPLC marketing functions. He received his Ph.D. degree in chemistry in 1995 from Saarland University in Saarbruecken,
Germany, where he became assistant professor in 1997 and associate professor in 2003, before moving into the industry.

Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.












