A Rapid and Sensitive LC–MS–MS Method for the Analysis of Three Forms of Thyroid Hormones Using Raptor™ Biphenyl LC Columns
The Application Notebook
Shun-Hsin Liang, Paul Connolly, and Ty Kahler, Restek Corporation
Thyroid hormones are essential for the regulation of development and growth in humans and animals. The thyroid gland produces thyroxin (T4) and tri-iodothyronine (T3) and quickly releases these compounds into the circulatory system. The concentration of circulating T4 is 50–60 times higher than T3 and the majority of these molecules are bound to blood proteins. The unbound or “free” T3 and T4 are the active forms of the hormone which only represent a small portion (less than 1%) of total thyroid hormones. Accurate and sensitive measurement of low pg/mL levels of free hormones is necessary to assess thyroid function for both veterinary and human diagnostics. Reverse tri-iodothyronine (rT3) is an inactive form that results from T4 biotransformation. Since rT3 functions as the feedback inhibitor of thyroid hormone production, the measurement of rT3 can be an important diagnostic marker with clinical implications.
The intent of this application was to develop an LC–MS–MS method for the analysis of thyroid hormones at the free form levels using the highly efficient and selective Raptor™ Biphenyl LC column. The clinical applicability of this method was demonstrated by analyzing fortified thyroid hormone in phosphate buffered saline (PBS) containing 4% human albumin.
Experimental
Instrument and Analytical Conditions
The instrument and analytical conditions are listed in Table 1. The analyte MRMs are shown in Table 2.
Sample/Calibration Standard Preparation
Human albumin was dissolved in PBS solution to a final concentration of 4%. This solution was used to prepare calibration standards ranging from 2 to 400 pg/mL. Standard solutions (0.5 mL) were fortified with 5 µL of internal standard (T4-13C6 [1 ng/mL]) and mixed with 1 mL of acetonitrile in a 4 mL glass vial. A 2 mL aliquot of ethyl acetate was added, stirred for 2 min, and then centrifuged for 10 min at 4300 rpm. The organic phase was removed and placed into a 4 mL glass vial, then evaporated to dryness at 55 °C under a gentle stream of nitrogen. The dried extract was reconstituted with 80 µL of a 30:70 water:methanol solution and injected (10 µL) into the LC–MS–MS for analysis.
Results
Chromatographic Separation of Thyroid Hormones
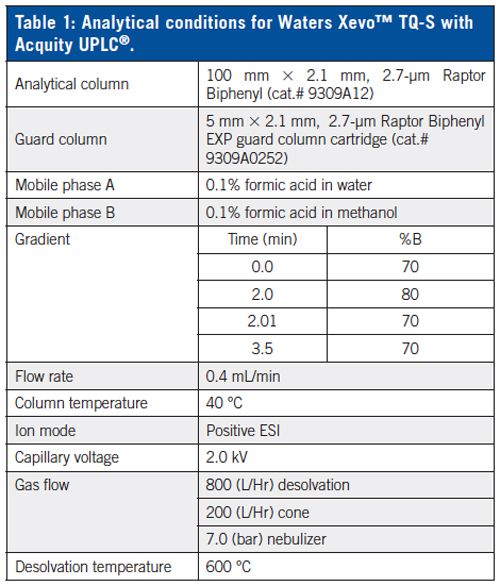
Since the most sensitive mass transitions for T3 and rT3 are identical, it is necessary to chromatographically separate these two compounds for accurate quantitation. With independent injections of T3 and rT3, it was shown that T3 and rT3 were completely resolved with the Raptor™ Biphenyl column (Figure 1). An example chromatogram (Figure 2) shows the baseline resolution of three forms of thyroid hormone from extracted sample (20 pg/mL) within 2 min.
Figure 1: Chromatographic separation of T3 and rT3 on the Raptor™ Biphenyl column. Note peak separation is critical for accurate analysis because these compounds have identical transitions and cannot be distinguished by MS–MS alone.
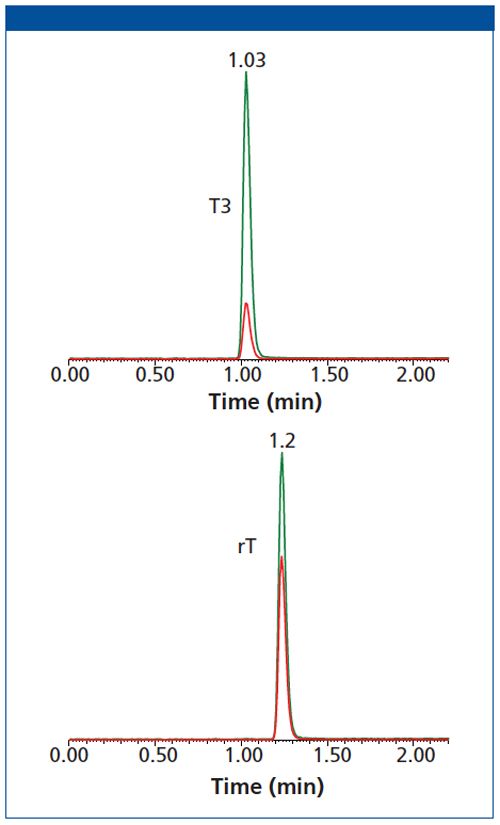
Figure 2: Complete chromatographic separations of all three extracted thyroid hormones (20 pg/mL) are obtained in less than 2 min. Peak ID: 1. T3, 2. rT3, 3. T4, 4. T4-13C6.
Linearity
Good linearities (1/x weighted) were obtained for all three forms of thyroid hormones with coefficient of determination (R2) values
> 0.990 from 2 to 400 pg/mL (for T3) or 5 to 400 pg/mL (for T4 and rT3). The %deviation was < 15%.
Conclusions
The Raptor™ Biphenyl column is excellent for rapid and sensitive analysis of thyroid hormones. With the method described here, concentrations of thyroid hormones as low as 2 pg/mL (T3) or 5 pg/mL (T4 and rT3) can be determined with less than 3.5 min of total analysis time. The analytical method is thus applicable to the clinical analysis of free thyroid hormone at low pg/mL levels.

Restek Corporation
110 Benner Circle, Bellefonte, Pennsylvania 16823, USA
Tel: (800) 356 1688 fax: (814) 353 1309
Website: www.restek.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.













