Comprehensive Analysis of Tobacco Smoke Using GC×GC–TOF-MS with Tandem Ionisation
The Application Notebook
Laura McGregor, Matt Edwards, Stefan Koschinski, and David Barden, Markes International
In this study, we demonstrate the enhanced separation and unparalleled sensitivity that can be achieved in the characterization of cigarette smoke using thermal desorption (TD) and GC×GC with time-of-flight mass spectrometry, aided by Tandem Ionisation for simultaneous acquisition of hard and soft electron ionisation (EI) data.
The Composition of Cigarette Smoke
The hazardous constituents of cigarette smoke have attracted considerable attention lately, especially with increasing regulation around the world limiting or banning smoking in public places - and even in private cars if children are present.
From an analytical perspective, however, there is much that remains to be learnt about the composition of cigarette smoke, because of its high degree of complexity - tobacco smoke is thought to contain thousands of components across multiple chemical classes and wide concentration ranges.
Comprehensive two-dimensional gas chromatography (GC×GC), when coupled with time-of-flight mass spectrometry (TOF-MS), is a powerful approach for the analysis of complex samples, improving chemical fingerprinting in areas of study as diverse as petrochemical analysis and fragrance profiling.
In this study, we use flow-modulated GC×GC–TOF-MS to enable smoke constituents to be routinely and confidently sampled, separated, and identified. The use of Tandem Ionisation® is also harnessed to increase the analytical resolution of the system, by providing both reference-quality 70 eV spectra and soft electron ionisation (EI) spectra simultaneously in a single analysis.
Experimental Procedure
Cigarette smoke (~50 mL) was drawn (on the fourth “puff”) directly onto an inert-coated Tenax® TA sorbent tube using an ACTI-VOC™ low-flow pump (Markes International).
Analysis of the tubes was performed by TD–GC×GC–TOF-MS, using a TD100-xr™ thermal desorber (Markes International), a BPX5 1st-dimension column, a DB17MS 2nd-dimension column, an Insight™ flow modulator (SepSolve Analytical), and a BenchTOF-Select™ mass spectrometer (Markes International).
TOF-MS instrument control and data acquisition used TOF-DS™ (Markes International), and GC×GC data visualization and processing used the ChromSpace® plug-in (Markes International).
Characterization of Tobacco Smoke
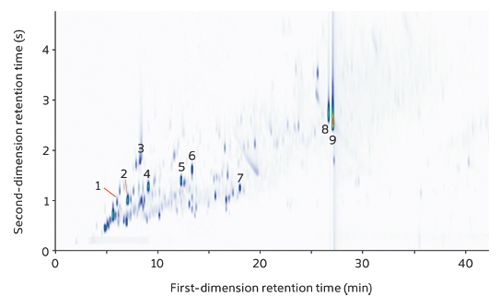
The GC×GC–TOF-MS plots of the three collected smoke samples showed the same major components, with nicotine and triacetin unsurprisingly the dominant peaks. Sample A is shown as an example (Figure 1).
Figure 1: GC×GC–TOF-MS colour plot for tobacco smoke from a commercial cigarette brand. Note the minimal breakthrough and tailing even for the most abundant compounds (nicotine and triacetin). 1, Benzene; 2, 2,5-Dimethylfuran; 3, Propylene glycol; 4, Toluene; 5, m- and p-Xylene; 6, o-Xylene; 7, Limonene; 8, Triacetin; 9, Nicotine.
Enhanced GC×GC Separation
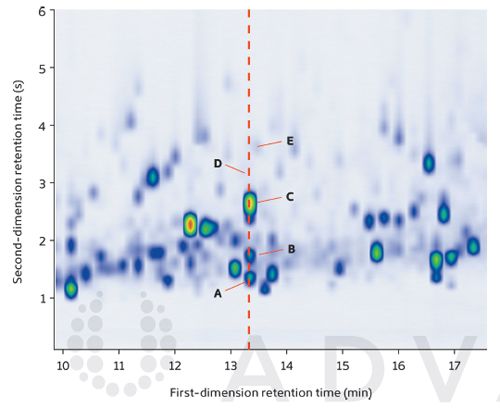
The expanded region shown in Figure 2 shows the efficient modulation and excellent separation provided by this GC×GC method, with average peak widths of ~200–300 ms in the second dimension. At one point, at least five components would be expected to co-elute in a one-dimensional separation (dotted red line), which would likely have resulted in identification difficulties. Here, however, confident matches against the NIST 14 database were obtained in every case.
Figure 2: Expanded region of the GC×GC–TOF-MS tobacco smoke colour plot, showing the separation capacity of flow-modulated GC×GC–TOF-MS. NIST 14 forward & reverse spectral matches were: (A) Non-1-ene, 905 & 908; (B) 2,6-Dimethylhepta-1,3,5-triene, 870 &877; (C) Styrene, 893 & 896; (D) 2,3-Lutidine, 758 & 813; (E) Cyclohexanone, 805 & 850.
Tandem Ionisation
Although the “reference-quality” spectra generated by BenchTOF enable the confident characterization of cigarette smoke described above, challenges may still remain in cases where compounds exhibit similar spectral characteristics at conventional 70 eV ionisation energy.
Markes’ Tandem Ionisation technology addresses this problem by multiplexing two ionisation energies within a single analysis, and was applied here at 70 eV and 14 eV EI. This created two independent data files with the obvious advantage of eliminating the need to collect a duplicate sample, and results in two perfectly aligned chromatograms from a single analytical run, simplifying both the acquisition and processing of soft-ionisation data.
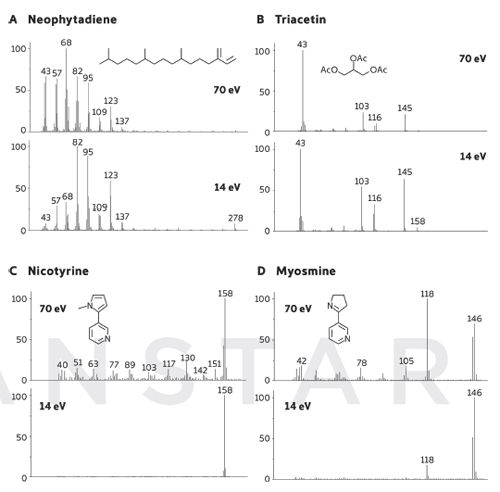
In tobacco smoke analysis, the availability of complementary soft EI spectra can be of considerable value, as illustrated by the spectra in Figure 3. Neophytadiene is a particularly interesting example because this diterpene is present at low levels in green tobacco leaf, but it is known to increase in concentration during yellowing and curing. Neophytadiene undergoes a high degree of fragmentation at 70 eV, making confident identification problematic, but acquiring data at 14 eV using Tandem Ionisation enhances the structurally-significant peaks, enabling its identity to be verified. Triacetin is the most extensively used plasticizer in cigarette filters, but the significant ions in the 70 eV spectrum have very low intensity. Tandem Ionisation provides enhancement of the higher-m/z ions (such as m/z 145 and 158) for simpler identification of this compound in complex matrices, and making it possible to achieve reliable quantitation using these diagnostic ions, rather than the more common m/z 43.
Figure 3: Comparison of 70 and 14 eV spectra for four compounds in tobacco smoke, obtained by Tandem Ionisation.
Conclusions
In this study, we have shown that flow modulation for GC×GC can provide excellent separation of trace-level analytes while minimizing breakthrough and tailing of highly concentrated compounds. Furthermore, Tandem Ionisation using BenchTOFâSelect provides another level of confidence in compound identification, through the simultaneous acquisition of NIST-quality 70 eV spectra and complementary soft EI spectra with enhanced molecular (and other structurally-significant) ions.
When these GC×GC–TOF-MS technologies are combined with the sensitivity enhancement possible with thermal desorption, the result is a highly-automated platform with a high degree of analytical resolution. As demonstrated here, such capabilities make it very well-suited for tackling the analytical challenges encountered in the tobacco industry, whether in matters of regulatory compliance or research and development.

Markes International
Gwaun Elai Medi-Science Campus, Llantrisant, Wales, UK
Tel.: 44 (0)1443 230935
E-mail: enquiries@markes.com
Website: www.markes.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.













