Light Scattering for the Masses® Monoclonal Antibodies and the Preparative Use of Flow Field-Flow Fractionation (AF4)
A.J. Freitag, K. Wittmann, G. Winter, and J. Myschik, Wyatt Technology Corporation
Proteins - especially monoclonal antibodies (MABs) - have become increasingly important in pharmaceutical work. However, there are some important differences between conventional, chemically-synthesized drugs and proteins. Because of the complex and weak structure of proteins, even a slight change in conditions, such as pH value, temperature, or mechanical stress, may lead to aggregation and a loss of activity or stability.
A separation of MABs before analysis is preferred because a detailed investigation of a fraction would be much easier and provide a better insight into the physical and chemical properties, compared to an analysis in the presence of a coexisting monomeric protein.
Asymmetrical flow field-flow fractionation (AF4) is a well-established method for sizing and quantifying different aggregate species in protein formulations. A major advantage of AF4 is the use of the formulation buffer of the protein as the mobile phase. The separation is independent of the ionic strength of the buffer.
The capability of AF4 as a preparative tool for the separation of aggregates, fragments, and monomeric species was investigated in this study. The system consisted of an Eclipse (Wyatt Technology Europe GmbH, Dernbach, Germany), equipped with DAWN multiâangle light scattering (MALS), refractive index (RI) and ultraviolet (UV) detectors, and a fraction collector. The separation was achieved by using a semiâpreparative SP channel with 350 μm height.
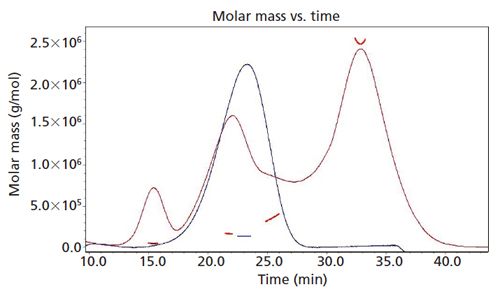
A monoclonal antibody sample was exposed to light, resulting in the formation of fragments and aggregates (Figure 1). A sample of 1 mg of protein per run was injected into the channel and collected in tubes made of glass, using a Gilson FC-203B fraction collector. The collected fractions were concentrated and the final concentration was determined by UV absorbance at 280 nm using a Micro-BCA Assay.
Figure 1: UV 280 nm fractograms and MALS detection of light exposed MAb1 (red line) and native MAb1 (blue line).
AF4 is a useful method for analytical and preparative separation of protein aggregates since the separation can be performed in each buffer or even pure water, thus facilitating subsequent activities. Furthermore, an investigation of possible immune responses triggered by protein aggregates in comparison to monomeric proteins can be performed, which is currently of major interest in administrative organizations and the pharmaceutical industry at large.
The results presented in this application note have been published before in A.J. Freitag, K. Wittmann, G. Winter, and J. Myschik, LCGC Europe24(3), 134–140 (2011).

Wyatt Technology Corporation
6300 Hollister Ave., Santa Barbara, California 93117, USA
Tel: +1 (805) 681 9009 fax: +1 (805) 681 0123
E-mail: info@wyatt.com
Website: www.wyatt.com

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.














