Rapid Analysis of Corn Stover Acid Hydrolysate for Estimation of Total Monosaccharide Content
Corn stover is the above-ground portion of the plant minus the kernels and it accounts for a large percentage of the global supply of lignocelluosic biomass available as feedstock for fermentation systems used for biofuel production.
Corn stover is the above-ground portion of the plant minus the kernels and it accounts for a large percentage of the global supply of lignocelluosic biomass available as feedstock for fermentation systems used for biofuel production. Acid hydrolysis of corn stover with sulphuric acid is a common method to release water-soluble carbohydrates for biofuel production. Monosaccharide concentrations released can vary with the feedstock, its pretreatment, hydrolysis and storage conditions. Knowledge of the monosaccharide content of acid-hydrolysed corn stover allows evaluation of the effectiveness of a new hydrolysis process and/or an estimation of the product yield.
Carbohydrates lack a good chromophore and, therefore, require high concentrations to be detected by UV absorbance or refractive index. Non-carbohydrate ingredients of acid-hydrolysed corn stover with good chromophoric properties can interfere with carbohydrate determinations. Pulsed amperometric detection (PAD) is selective for compounds that can be detected under a given set of electrochemical conditions and has a wide linear range for carbohydrates.
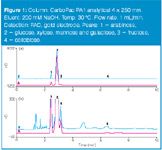
Figure 1
High-performance anion-exchange chromatography (HPAE) can separate glucose, galactose, arabinose, xylose, mannose, fructose, cellobiose, rhamnose, sucrose, mannitol and other carbohydrates typical of acid-hydrolysed plant-derived materials. The CarboPac PA1 anion-exchange column resolves monosaccharides and disaccharides from the unretained non-carbohydrate components using hydroxide eluent. Generally, at high hydroxide concentrations (e.g., 200 mM NaOH), monosaccharides elute rapidly and are poorly resolved. When the knowledge of the types of monosaccharides present in the acid-hydrolysed feed stock is not necessary, a rapid method that combines the total monosaccharides into one or two peaks for quantification may be more desirable.1 The method presented here allows estimation of total monosaccharides in undiluted corn stover acid hydrolysate in less than 10 min using HPAE-PAD.
Experimental
Dionex ICS-3000 chromatography system consisting of a pump, detector chromatography module (with electrochemical detector, gold working electrode with 15 mL PTFE gasket, 0.2 µL injection loop) and autosampler. The CarboPac PA1 column was used with 200 mM NaOH at 30 °C with a flow-rate of 1 mL/min. Corn stover acid hydrolysate was centrifuged at 16 k x g for 10 min to remove particulates, then directly injected.1
Results and Discussion
Figure 1 shows the chromatograms of undiluted corn stover hydrolysate (blue trace) and standards (magenta trace). Panel B reveals greater detail. Arabinose (peak 1) is resolved from galactose, glucose, mannose and xylose (peak 2) and fructose (peak 3). The combined peak area for peaks 1, 2 and 3 is an estimate of the monosaccharide content of this sample.
Reference
1. Dionex Corporation. Rapid Method for the Estimation of Total Free Monosaccharide Content in Corn Stover Hydrolysate Using HPAEPAD. Application Note 225, LPN 2190. Sunnyvale, California, USA.
CarboPac is a registered trademark of Dionex Corporation.

Dionex Corporation
1228 Titan Way, PO Box 3603, Sunnyvale
California 94088, USA
tel. +1 408 737 0700 fax +1 408 730 9403
Website: www.dionex.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













