Ion Chromatographic Determination of Free and Total Glycerol in Biodiesel and Biodiesel Blends
The free and total content of detrimental glycerol in vegetable oil methyl esters (biodiesel) is of paramount importance for the quality of biodiesel and is, therefore, limited by the US ASTM D 6751 and the European EN 14214 standards. Both regulations currently stipulate gas chromatographic (GC) analysis of free and total glycerol. However, the GC method, apart from being expensive, requires tedious derivatizations and fails for glycerol determinations in coconut or palm kernel oil methyl esters.
J. Gandhi,1 A. Wille2 and A. Steinbach,2
1 Metrohm USA Inc., Riverview, Florida, USA,
2 Metrohm International Headquarters, Herisau, Switzerland.
The free and total content of detrimental glycerol in vegetable oil methyl esters (biodiesel) is of paramount importance for the quality of biodiesel and is, therefore, limited by the US ASTM D 6751 and the European EN 14214 standards. Both regulations currently stipulate gas chromatographic (GC) analysis of free and total glycerol. However, the GC method, apart from being expensive, requires tedious derivatizations and fails for glycerol determinations in coconut or palm kernel oil methyl esters.
In contrast, the presented ion chromatographic method is applicable to all types of vegetable oil methyl esters. Prior to chromatographic separation, free glycerol and total glycerol are isolated by a straightforward extraction and saponification-extraction technique, respectively. Pulsed amperometric detection following chromatographic separation achieves an outstanding method detection limit (MDL) of 0.7 ppm by mass for glycerol and thus easily fulfils ASTM and EN performance specifications.
Introduction
The four primary driving forces behind biofuels are the world's increasing thirst for petroleum (80 Mbarrels/day), the diminishing supply of fossil fuels, global warming and the intention to reduce the dependence on fuel imports. Additionally, most biofuels are produced by straightforward manufacturing processes, are readily biodegradable and nontoxic, have low emission profiles and can be used as is or blended with conventional fuels.1
Biodiesel is produced by transesterifying the triglycerides in the parent oil or fat with an alcohol, usually methanol, in the presence of a catalyst (base, acid or enzyme) to yield fatty acid methyl esters (FAME) and free glycerol as co-product (Figure 1). Because reaction rates under acid or enzyme catalysis are relatively slow, most producers use the rapid alkali-catalysed transesterification.

Figure 1
Incomplete reaction leads to the formation of residual glycerol intermediates such as mono-, di- and triacylglycerides (bonded glycerols). In contrast, complete conversion results in the formation of highly water-soluble glycerol (free glycerol). The latter is separated from the final product at the end of the production process. However, traces of glycerol are frequently found in the ester phase. Both free and bonded glycerols (= total glycerol) lead to severe operational problems such as injector and valve deposits or filter clogging. Accordingly, the US ASTM D 67512 and the European EN 142143 specify a maximum total glycerol content of 2400 ppm (0.24%) and 2500 ppm (0.25%), respectively. The maximum free glycerol content is limited to 200 ppm (0.02%) in both standards, which stipulate gas chromatographic (GC) analysis involving time- and reagent-consuming derivatizations. In addition, GC methods still encounter coelution problems with biodiesel samples produced from or containing lauric oils, such as coconut and palm kernel oil. Moreover, the GC method is not applicable to biodiesel blends.
Based on the analysis of biodiesel blends made from coconut oil, this paper demonstrates sensitive analysis of the free and total glycerol content via simple and innovative ion chromatography followed by pulsed amperometric detection (PAD).
Experimental
Instrumentation
The chromatography system (Metrohm AG) consisted of the 871 Advanced Bioscan, 818 Advanced IC Pump and 838 Advanced IC Sample Processor (Figure 2). For all separations, a Metrosep Carb 1 – 250 anion exchange column was used with a flow rate of 1 mL/min. The injection valve was fitted with a 10 µL injection loop and separation was achieved by isocratic elution employing a 100 mmol/L NaOH eluent.

Figure 2
The electrochemical detector consists of a gold working electrode in combination with a solid-phase reference electrode and a stainless-steel auxiliary electrode. A triple-step potential waveform is applied4 with the following pulse potentials and durations: E1 = 50 mV (t1 = 400 ms), E2 = 750 mV (t2 = 200 ms) and E3 = –150 mV (t3 = 400 ms). The output range of the detector was set to 5 µA.
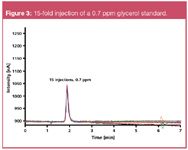
Figure 3
Instrument control, data acquisition and processing were performed by Metrodata IC Net software (Metrohm).
Reagents
Glycerol standard and potassium hydroxide were reagent grade and purchased from Sigma-Aldrich (Milwaukee, Wisconsin, USA). Synthetic biodiesel blends B2 to B20 were produced by respective mixing of biodiesel made from glycerol-containing coconut-oil and low-sulphur petroleum diesel. All standard solutions and eluents were prepared from deionized water with a specific resistance higher than 18 MΩ·cm.

Table 1
Extraction and saponification
While the extraction of free glycerol can be performed by a simple separating funnel, saponification-extraction for bound glycerol requires a commercially available reflux system capable of heating the reaction mixture to 90 °C.
(a) Free glycerol
Generally, a high free glycerol content points to incomplete separation of the ester and glycerol phase.5 Because of the high water solubility of the triol — 1000 mg and more will dissolve in a litre of water — free glycerol can be readily extracted from biodiesel or biodiesel blends.
The procedure comprises the addition of 45 g of distilled water to approximately 5 g of sample. After vigorous shaking for five minutes the sample is allowed to stand for another five minutes. After phase separation, an aliquot of the aqueous phase is filled into a chromatography vial and placed on the sample processor for analysis.
(b) Total glycerol
Total glycerol is the sum of free and bound glycerol. The latter is the sum of residual mono-, di- and triglycerides and stems from incomplete esterification reactions. Glycerides are removed from the organic phase by saponification reaction with sodium hydroxide and subsequent extraction of the generated glycerol with water.
In a reflux system, 20 mL of 0.01 mol/L potassium hydroxide is added to approximately 2 g of sample. The mixture is heated to reflux for one hour. After cooling to room temperature, the volume of the mixture is made up to 50 mL. The released glycerol is then extracted into the aqueous phase according to the procedure described above.
Results
Calibration and method detection limit
Calibration standards range from 0.5 to 100 mg/L. Calibration is linear providing a correlation coefficient of 0.99996 with a relative standard deviation better than 0.437%.
The detection limit was determined by a 15-fold injection of a 0.7 ppm glycerol standard. The excellent MDL of 0.7 ppm glycerol by mass (0.7·10–4 %) exceeds by far the maximum free glycerol content of 200 ppm (0.02%) required by the ASTM D 6751 or EN 14214.
Free and total glycerol content in different biodiesel blends and a coconut-oil biodiesel sample
Whereas the total glycerol limit in ASTM D 6157 and EN 14214 is 0.24% and 0.25%, respectively, the allowed maximum content of free glycerol is only 0.02% in both standards. The pure biodiesel sample B100 contains 0.027% of free and 0.62% of total glycerol and thus exceeds the limits stipulated by ASTM D 6751 and EN 14214. Before being used as fuel or being blended with petroleum diesel, the coconut methyl ester first has to be freed of its excess glyceride and glycerol contents.
According to Table 1 and Figure 4 the free and total glycerol contents in all investigated biodiesel blends B2 to B20 are below 0.0051% and 0.124%.

Figure 4
Conclusion
The presented method demonstrates a simple and innovative approach for the analysis of free and total glycerol in biodiesel and biodiesel blends. In contrast to the GC method stipulated in the American ASTM D 6751 and European EN 14214, IC even works with coconut oil requiring only a straightforward extraction for free glycerol and saponification-extraction for bound glycerol before chromatographic separation. With an MDL of 0.7 ppm by mass for total glycerol, IC-PAD easily exceeds the requirements of ASTM and DIN standards.
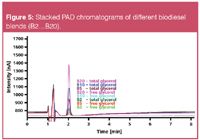
Figure 5
References
1. A. Steinbach et al., Supplement to Petroleum Technology Quarterly (PTQ), Biofuels Plant & Technology, Quality control of biofuels, 21–29 (2008).
2. ASTM D 6751, Standard specification for biodiesel fuel blend stock (B100) for middle distillate fuels.
3. DIN 14214, Automotive fuels — fatty acid methyl esters (FAME) for diesel engines — requirements and test methods.
4. A. Steinbach and A. Wille, G.I.T. Laboratory Journal, 5–6, 32–34 (2009).
5. K. Knothe, J. Van Gerpen and J. Krahl, The Biodiesel Handbook, AOCS Press, 304 pages (2005).
6. J. Gandhi, W. Donaldson and R. Benton, Simple and innovative methodology for determination of glycerol in biodiesel and biodiesel blends (B2-B100) by ion chromatography, Pittcon 2008 (downloadable under http://products.metrohm.com, search for 8.000.6008EN).

Metrohm International Headquarters
CH-9101 Herisau, Switzerland
tel. +41 71 353 85 04
E-mail: aw@metrohm.com
Website: www.metrohm.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













