Quick Polar Pesticides (QuPPe): Learning From and Expanding Upon Others
In January, Steve Lehotay from the U.S. Department of Agriculture provided an update on the quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction method, originally developed for multiresidue pesticide analysis. Like any method, QuEChERS had some deficiencies, which were addressed with the new QuEChERSER (adding “efficient” and “robust” to the acronym) mega-method. Meanwhile, the European Union (EU) Reference Laboratories developed a new method for the multiresidue analysis of highly polar pesticides, called the Quick Polar Pesticides (QuPPe) method. This month, we take a look at QuPPe, comparing and contrasting it with QuEChERS, and noting how we can learn from previously developed methods as we strive for improvements.
Steve Lehotay (1) engaged us with a summary of the current state of the quick, easy, cheap, effective, rugged, and safe (QuEChERS) multiresidue extraction method in our last “Sample Preparation Perspectives” column. Lehotay and Anastassiades developed QuEChERS approximately 20 years ago as a multiclass, multiresidue method, primarily for determining pesticides in produce. Because the method developed employed a wide range of flexibility (that is, it was simple and developed for the analytical instrumentation widely used at the time), modifications to the approach led to it becoming a model for the extraction and chromatographic sample preparation of more diverse analysis needs, such as for veterinary drugs, environmental contaminants, and mycotoxins. The still growing popularity of QuEChERS is evidenced by close to 4500 peer-reviewed publications since its inception.
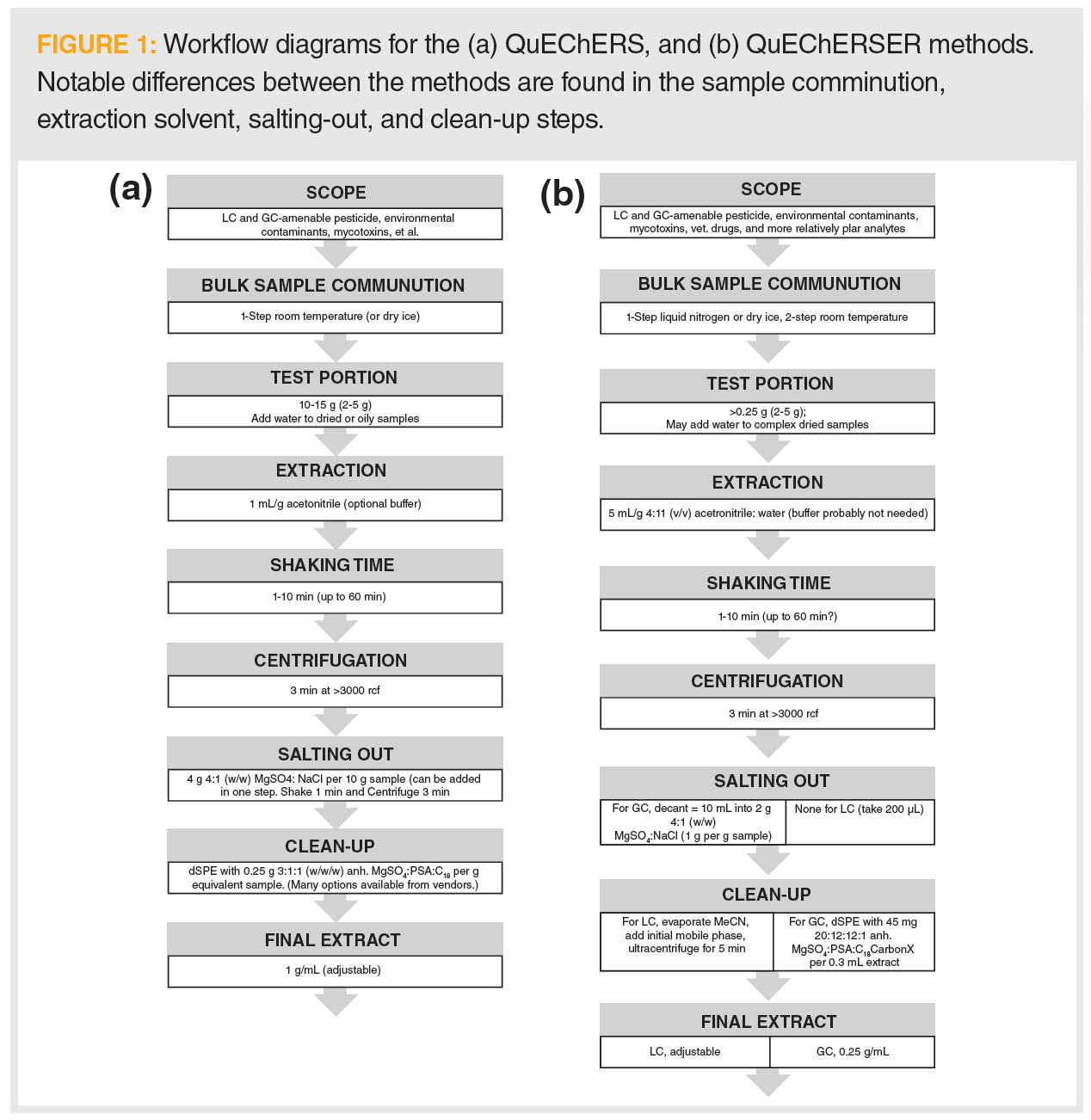
The simplicity and flexibility inherent in the QuEChERS method also results in its limitations. For example, the minimal or “just enough” clean-up steps avoiding indirect matrix effects make the approach both applicable as a template for an array of analyses and provide some limits on the range of residue polarities amenable to the technique. Additionally, analytical instrumentation, such as liquid chromatography–tandem mass spectrometry (LC–MS/MS), has improved dramatically since the turn of the century. With this in mind, Lehotay updated the technique to include “efficient and robust”, and updated the method name to QuEChERSER, which was the primary topic of the January column (1). The QuEChERSER upgrade accommodates a broader scope of polar and nonpolar analytes, creating what Lehotay describes as a mega-method. A comparison of the workflows for QuEChERS and its offspring QuEChERSER is shown in Figure 1. From these schematics, the major differences between the methods can be gleaned.
Quick Polar Pesticide Extraction (QuPPe)
Despite the improvements afforded by QuEChERSER, there is still a need for the simple and effective extraction of residues of polar pesticides incurred in produce and other foodstuff. Working with the European Union (EU) Reference Laboratories for Residues of Pesticides, Michelangelo Anastassiades, co‑inventor of the original QuEChERS method, created a new method that was aimed specifically at the highly polar pesticides not amenable to extraction via QuEChERS (or QuEChERSER) (2), which he called the Quick Polar Pesticide (QuPPE) extraction. The workflows for the QuPPe family of methods is shown in Figure 2. Although starting with goals similar to those used with the original QuEChERS extraction, what is immediately evident in these workflows is that Anastassiades learned and reapplied concepts from the previous development of QuEChERS and added his knowledge of chemistry in the development of QuPPe.

A series of four QuPPe methods are described, one each for most plant-based commodities and honey; cereals, pulses, nuts, and oily seeds; liver, kidney, muscle, and milk; and animal fat. In these methods, the sample is weighed and water may also be added. The acidified method is used for the extraction, and heating (fats) or cooling (lipids and proteins) is used to remove biological macromolecules. The clean-up process is performed prior to LC–MS/MS or ion chromatography (IC)–MS/MS. Ethylenediaminetetraacetic acid (EDTA) is added, as necessary, to the complex metal ions. Isotopically labelled internal standards are added at the beginning of the procedures to compensate for volume deviations, analyte loss, or matrix effects on recovery rates. Where internal standards are not possible, quantification by standard addition is used. Because of the highly polar nature of the target analytes, plastic vials are used to minimize loss of analyte because of surface adsorption onto glass vials, though some carryover is still possible depending on the analyte. Because of the simplicity of the method, strong matrix effects are frequently observed. Prior to QuPPe, samples are ground to less than 500 μm and homogenized to a free-flowing mixture.
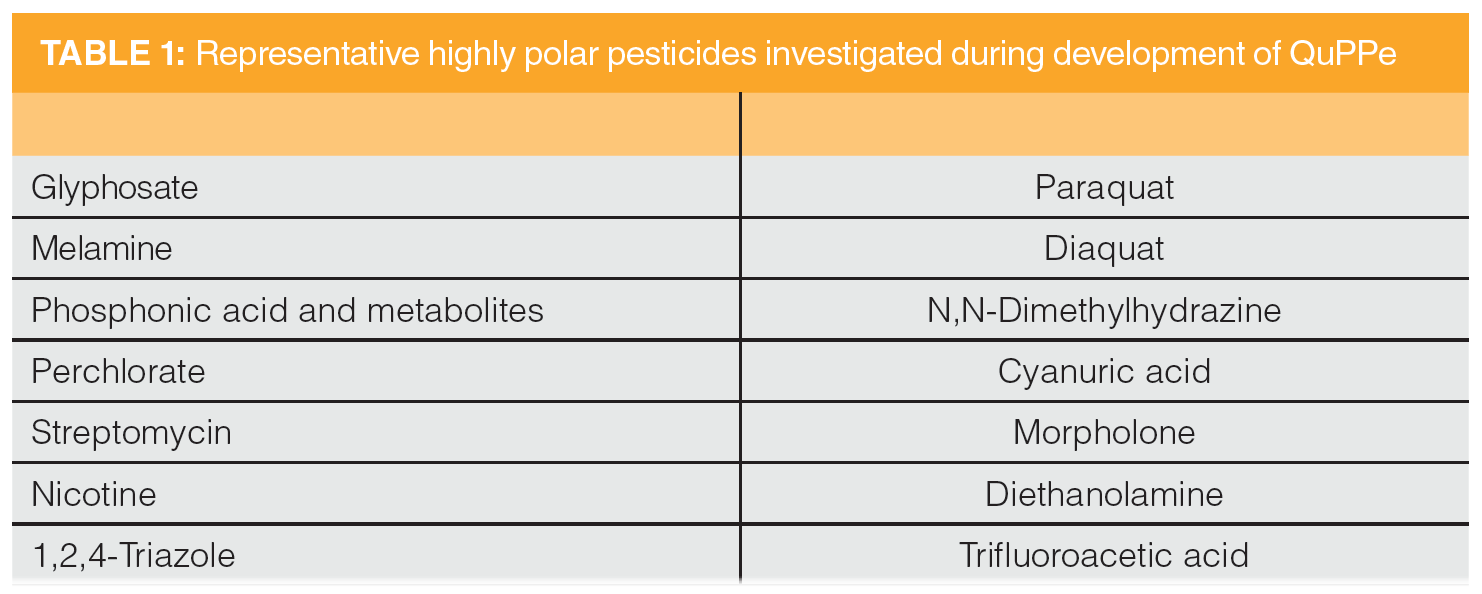
During the development of QuPPe, the analytical team spiked a host of highly polar pesticides (Table 1) at levels of 0.005–20 mg/kg per sample and conducted matrix-matched and matrix isotope-labelled internal standards (ILIS) calibrations with interlaboratory calibrations. The mean recoveries reported were between 70–120%, with a 0–20% relative standard deviation. Spiked samples on to a number of foods of plant- and animal-origin were reported, including citrus fruits, pome fruits, stone fruits, soft and small fruits, dried fruit, root and tuber vegetables, leek plants, fruiting vegetables, cabbages, leafy vegetables and herbs, stem vegetables, legumes or pulses, cereals, oily seeds, nuts, animal tissues, whole fat and skimmed milk, honey, eggs, and animal fats.
Applications of QuPPe
Given the relative newness of the method, reports of QuPPe are just starting to emerge, though the method is accepted by the EU. Perusal of the Scopus database shows 21 literature reports of QuPPe from 2016 through the end of 2021, with two to six papers per year.
One of the first literature reports (3) of glufosinate in samples of plant origin used multiwalled carbon nanotubes for cleanup, and reported linearity from 10–500 μg/kg for 12 sample matrices, with detection limits of 0.3–3.3 μg/kg. In one manuscript (4), a fusion of the QuPPe and QuEChERS methods was reported for the isolation of 45 nonpolar and polar residues, with a broad range of log Kow (over 10 orders). A parallel, orthogonal hydrophilic interaction liquid chromatography (HILIC) and reversed‑phase LC approach was used. Elsewhere, HILIC was observed to provide good retention and selectivity of highly polar herbicides extracted from plant-based foods (5). Importantly, fewer matrix effects were found when cleanup was performed with chitosan or graphene. Another report (6) added the primary‑secondary amine (PSA) during the clean-up step and used direct analysis in real time (DART)–MS, comparing orbital trap MS and quadrupole time-of-flight (QTOF) mass analyzers, for the analysis step.
Conclusion
Although still an emerging technique, the lessons learned from QuEChERS were re-applied in developing a simple method for the extraction of highly polar pesticides from samples of plant and animal origin. This method, and potentially modified versions, bears watching as an important tool for multiresidue monitoring.
References
- S.J. Lehotay, LCGC Europe 35(1), 26–31 (2022).
- M. Anastassiades, A.-K. Wachtler, D.I. Kolberg, E. Eichhorn, H. Marks, A. Benkenstein, S. Zechmann, et al., “Quick Method for the Analysis of Highly Polar Pesticides in Food Involving Extraction with Acidified Methanol and LC- or IC-MS/MS Measurement”, version 12 (published on EURL-SRM website on 23 July 2021). URL: https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=887&LabID=200&Lang=EN
- Y. Han, L. Song, P. Zhao, Y. Li, N. Zhou, Y. Qin, X. Li, and C. Pan, Food Chem. 197, 730–738 (2016).
- J. Robles-Molina, B. Gilbert-López, J. F. García-Reyes, and A. Molina-Díaz. J. Chromatogr. A 1517, 108–116 (2017).
- P. Kaczyński, Food Chem. 230, 524–531 (2017).
- F.J. Lara, D. Chan, M. Dickinson, A.S. Lloyd, and S.J. Adams, J. Chromatogr. A 1496, 37–44 (2017).
About The Column Editor
Douglas E. Raynie is Department Head and Associate Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high-resolution chromatography, and bioprocessing in supercritical fluids. Raynie is a member of LCGC’s editorial advisory board. Direct correspondence about this column via e-mail to amatheson@mjhlifesciences.com

Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)







