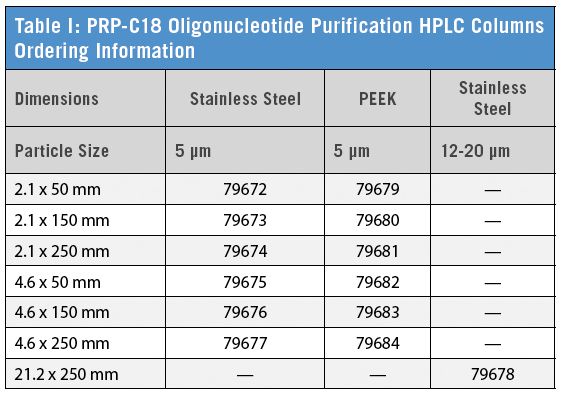
PRP-C18 Oligonucleotide Purification HPLC Columns
The Application Notebook
Hamilton's PRP-C18 is the First Choice for Oligonucleotide Purifications
Synthetic oligonucleotide (oligo) purifications are demanding applications that are typically performed at elevated (>60 °C) temperature in order to facilitate resolution between the target oligo and closely-related failure sequences. Traditional silica-based C18 (ODS) columns have diminished lifetime at elevated temperatures, however, polymeric polystyrene-divinylbenzene (PS-DVB) columns are routinely used to purify synthetic oligonucleotides due to their superior temperature (>100 °C) and pH (1–14) stability.
The Hamilton PRP-C18 is a next generation polymeric column based on porous C18-functionalized PS-DVB that exhibits excellent chemical and thermal stability. Unlike other polymer columns, the octadecylated surface modification imparts superior mass transfer kinetics for high efficiency separations and excellent mechanical stability.
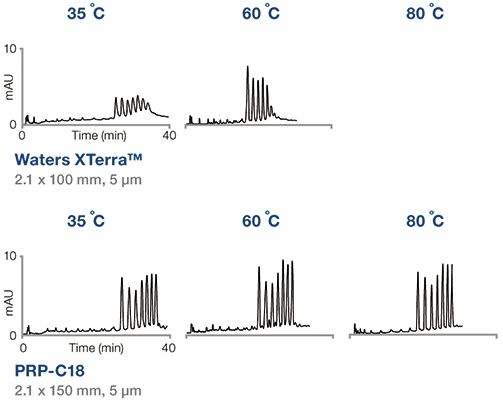
Separation of oligos at increasing temperatures.
Rugged Design Ensures More Oligo Manufacturing
The flexibility to employ elevated temperatures (up to 100 °C) and alkaline pH are important tools in oligonucleotide purifications. Traditional silica-based supports break down at elevated temperatures, leading to stationary phase bleeding and diminished column lifetimes. The PRP-C18 does not dissolve, phase-strip, or bleed even under the most extreme operating conditions, unlocking the power of superior resolution between long oligos and their failure sequences. Because the PRP-C18 stationary phase is devoid of silanols found in silica-C18 columns, peak tailing, poor recovery, and carry-over problems are completely eliminated, offering significant value.
Benefits
- Superior resolution for purer oligos
- Extended column lifetime for increased productivity
- High loading capacity for time and cost savings
Full pH Range Compatibility
Unlike traditional silica-based C18 columns, the PRP-C18 is stable over a pH range of 1–14. The flexibility to employ alkaline mobile phase (pH 12) is useful for separating problematic oligos that form secondary structures or aggregates.

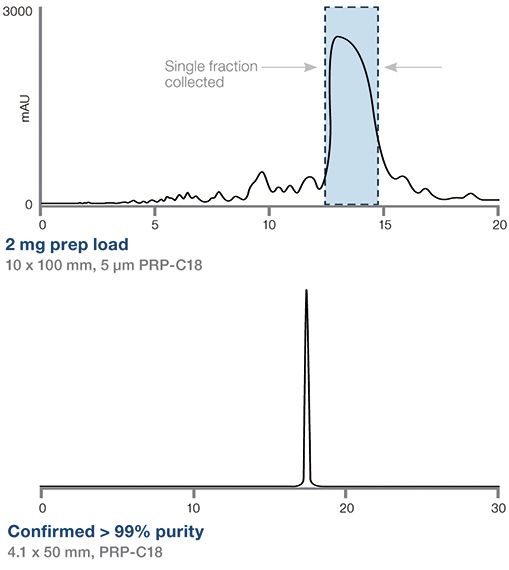
Higher Oligo Yield with Extended Loading Capacity
The high loading capacity and greater resolving power of the PRP-C18 makes it possible to achieve baseline resolution between failure sequences and the target 21mer, which is collected in a single fraction. Subsequent analytical chromatography of the purified fraction indicate oligo purification to >99% (UV).
High Purity Oligos with High-Resolution Separations
The performance of the PRP-C18 and two leading oligo columns at various temperatures is shown in Figure 1. The PRP-C18 shows the best resolution of poly(dC)12-18 ladder at all temperatures.
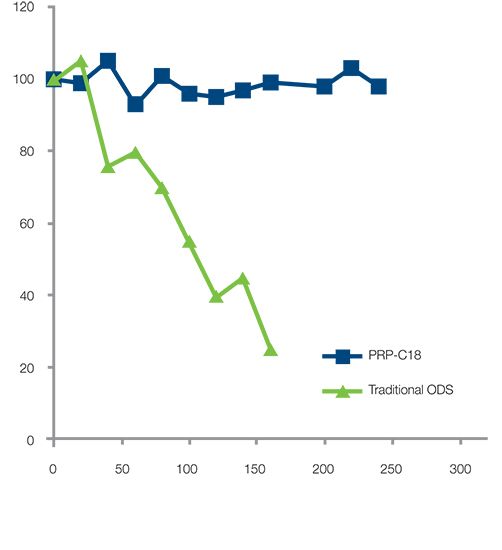
Figure 1: Forced degradation study comparing longevity of traditional ODS and Hamilton PRP-C18 columns under elevated temperature (60 °C) and alkaline pH (50 mM phosphate, pH 12) conditions.

Hamilton Company Inc.
4970 Energy Way, Reno, Nevada 89502
tel: +1-775-858-3000, fax: +1-775-856-7259
Website: www.hamiltoncompany.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)
















