Kinase Fragments Dimerize without Oligomerization Domains
The Application Notebook
Self-association is crucial for regulation of certain kinase proteins. However, a kinase fragment that lacks the oligomerization domain is still dimeric in solution, as determined by SEC-MALS.
Determination of oligomeric states is an important issue in protein chemistry. For example, self-assembly via oligomerization domains is crucial for the regulation of several protein kinases. Determination of the oligomeric state of fragments of these kinases is a means of verifying the involvement of each domain in self-assembly.
Analytical size exclusion chromatography (SEC) is widely used for determining molecular weight and oligomeric state of proteins in solution, but it exhibits some important limitations. For example, interactions of proteins with column material can lead to delayed elution and hence erroneous results when relying on column calibration. Since even ideal elution occurs according to hydrodynamic size rather than true molecular weight, there are no appropriate molar mass gel filtration standards for proteins, fragments, or complexes of non-globular structure that present a different size/molecular weight dependence than globular proteins. The use of size exclusion chromatography in combination with multiangle light scattering (SEC-MALS) determines molecular weights independently of elution time and conformation, overcoming the need for standards and the errors inherent in analytical SEC.
This note describes the analysis of a kinase fragment lacking its association domain in order to determine its oligomeric state in solution. SEC-MALS revealed that the kinase moiety clearly remains dimeric in solution, even in the absence of its purported oligomerization domain.
Experimental Conditions
An HP-SEC column was calibrated using bovine serum albumin (BSA) monomer and dimer. The kinase fragment and alcohol dehydrogenase (ADH, 38 kDa) were each run on the column, and the elution times compared to those of BSA monomer (66.4 kDa) and dimer (133 kDa). Absolute molar mass (MW) of the proteins at each elution volume were determined by analysis of signals from the multiangle light scattering and refractive index detectors (DAWN and Optilab, respectively, Wyatt Technology, Santa Barbara) in ASTRA software (Wyatt Technology). Chromatograms were overlaid with molecular weight values calculated for each elution time along the peaks, as seen in Figure 1.
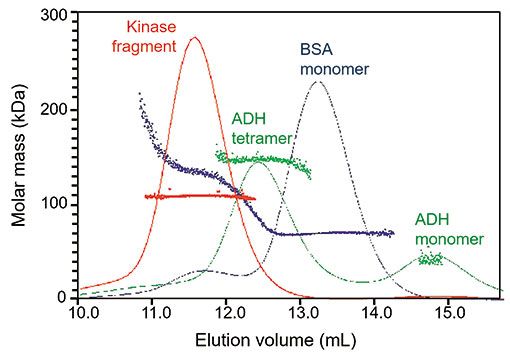
Figure 1: Molar mass, as determined by multiangle light scattering, versus elution volume of kinase fragment (red), bovine serum albumin (BSA, blue), and alcohol dehydrogenase (ADH, green). Molar masses deduced from the elution volumes of kinase fragment and ADH are shown to be misleading when compared with absolute molar masses from SEC-MALS
Results
The monomeric kinase fragment has a sequence molar mass of 53.5 kDa. The fragment (red trace) eluted at nearly the same volume as the BSA dimer (blue trace), suggesting that its molar mass is approximately 140 kDa, or trimeric. However, MALS determines an absolute molar mass in solution of 108 kDa, revealing that the protein is actually a dimer. The molecular weight is absolutely uniform across the peak, indicating a high degree of homogeneity. Such early elution is indicative of a non-globular conformation.
ADH tetramer (green trace, 150 kDa) eluted between the monomer and dimer of BSA, possibly because of ADH-column interactions that caused it to elute late relative to its size. Comparison of the fragment's elution volume to ADH monomer and tetramer would mislead the investigator to assume a tetrameric state, even further removed from the truth than comparison to BSA.
Conclusions
SEC-MALS provides true solution molecular weight for proteins, overcoming the inherent errors produced by reliance on column calibration. Here we have shown that kinase fragments are dimeric, even without the purported oligomerization domain; but they are not trimeric or tetrameric as might have been deduced via column calibration.

Wyatt Technology Corporation
6330 Hollister Avenue, Santa Barbara, CA 93117
tel. +1 (805) 681-9009, fax +1 (805) 681-0123,
e-mail: info@wyatt.com
Website: www.wyatt.com














