Postnova - Separation and Sizing of a Virus Mixture Using Asymmetrical Flow Field‐Flow Fractionation Coupled to Multi‐Angle Light Scattering
Biogen and Postnova present data on the analysis of adeno-associated viruses (AAV) using Asymmetrical Flow Field-Flow Fractionation coupled to Multi-Angle Light Scattering detection (AF4-MALS). AAV are promising gene therapy delivery vehicles, whose efficacy may be negatively affected by the presence of viral aggregates. Due to its gentle separation and broad applicable size range, AF4-MALS is able to characterize AAV and their aggregates with high resolution and precision, thereby overcoming the drawbacks that column-based chromatography techniques often face when dealing with samples larger than 50 nm in size.
Viruses are increasingly used as gene therapy delivery vehicles due to their versatility and safety. They can be loaded with DNA or RNA and delivered to a specific location in the body to treat or cure a disease (1). One of the biggest challenges for manufacturing a homogeneous virus sample is the presence of viral aggregates, which negatively affect transduction efficiency, biodistribution, and immunogenicity (2). Due to their relatively large size, often over 50 nm in diameter, virus aggregates are challenging to separate and characterize by column-based chromatography techniques such as size-exclusion chromatography (SEC). In this application note, we present data on separation of a virus mixture using Asymmetrical Flow Field-Flow Fractionation (AF4) and measurement of their radius of gyration (Rg).
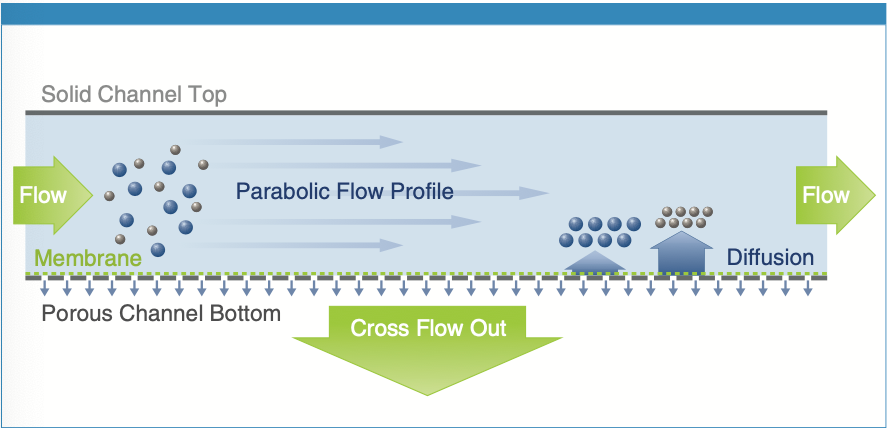
A schematic for the AF4 channel is shown in Figure 1. The combination of cross flow and channel flow enable size separation over the course of the analysis; small particles elute and reach the connected detectors before larger particles, including aggregates.
Figure 1: Schematic of the AF4 separation principle.
Experimental Details and Results
A virus mixture sample was created by combining smaller adeno-associated viruses (AAV) with larger adenovirus type 5 (Ad5) in solution to simulate a sample with virus monomer and aggregates. To separate the viruses by size, an AF4 (Postnova AF2000) was used, coupled to a Postnova 21-angle multi-angle light scattering (MALS, PN3621) detector for measuring the Rg. Both the AAV-only and virus mixture samples were analyzed by AF4-MALS to highlight the differences between the samples. The carrier solution was phosphate buffered saline (PBS). The AF4 membrane used was 10 kDa regenerated cellulose.
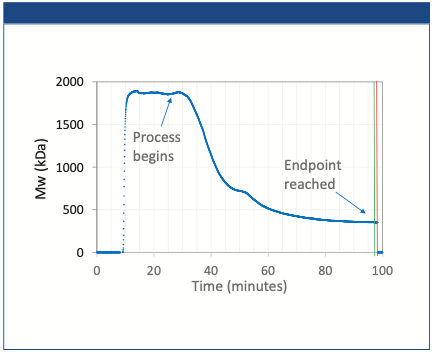
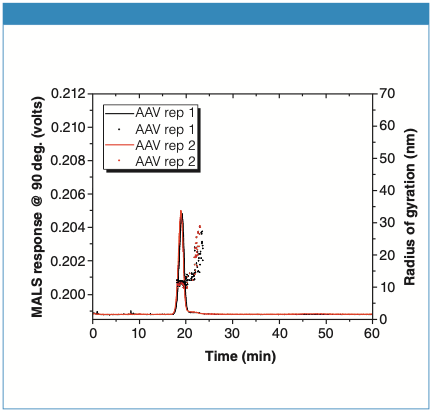
The MALS response for the AAV-only sample is shown in Figure 2. The main peak corresponds to the AAV monomer eluting between 17 and 20 min. The Rg measured for the monomer is ~ 12.5 nm, very consistent with an expected diameter of 25 nm. There is a small shoulder to the right of the monomer peak, and the increasing Rg for this shoulder peak indicates the presence of a small amount of dimer/trimer/ small aggregates in the sample. Fully separating the monomer from any aggregate species was beyond the scope of this work. Further method optimization is required to achieve this goal.
Figure 2: Analysis of AAV-only sample. The curves are replicate AF4-MALS fractograms, overlaid with Rg values (black and red dots).
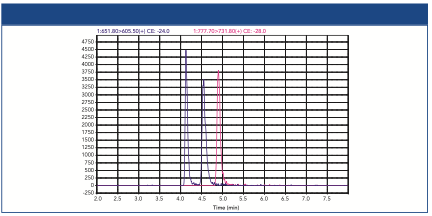
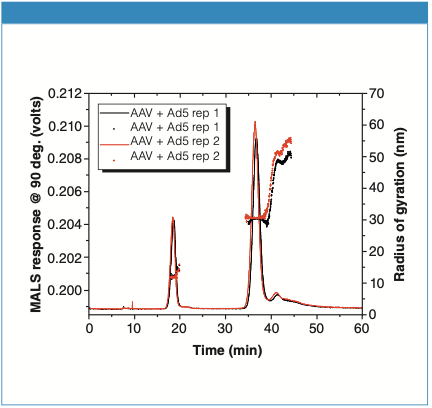
In Figure 3, the virus mixture is separated into multiple peaks, with the Rg plotted as black and red dots. The monomers have measured Rg values consistent with AAVs, at about 12.5 nm, with the slight shoulder (aggregates) still observed as in the AAV-only sample. A second peak between 33 and 40 min corresponds to the Ad5 virus, whereas a third peak that is eluted between 40 and 50 minutes are aggregates of the Ad5 virus. The Ad5 virus and its aggregates have radii in the range of 30–55 nm, most likely too large to be successfully separated by SEC.
Figure 3: Analysis of AAV and Ad5 virus mixture sample. The curves are replicate AF4-MALS fractograms, overlaid with Rg values (black and red dots).
Conclusion
The data presented here demonstrates that AF4-MALS is a powerful tool in the separation of virus particles. It can separate viruses and aggregates from a few nm up to >100 nm. This means that AF4-MALS can easily separate and size multi-modal virus samples, including the larger Ad5 virus and its aggregates with high resolution and precision.
References
(1) M.F. Naso, B. Tomkowicz, W.L. Perry III, and W.R. Strohl, BioDrugs 31(4), 317–334 (2017).
(2) J.F. Wright, T. Le, J. Prado, J. Bahr-Davidson, P.H. Smith, Z. Zhen, J.M. Sommer, G.F. Pierce, and G. Qu, Molecular Therapy 12(1), 171–178 (2005).

Postnova Analytics GmbH
86899 Landsberg, Germany
T: +49 8191 985 688 0
info@postnova.com
Website: www.postnova.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.