Positive-Negative Switching LC–MS–MS for Quantification of Pesticides in Juice
The Column
Liquid chromatography coupled to triple quadrupole tandem mass spectrometry (LC–MS–MS) in multiple reaction monitoring (MRM) mode is widely considered as the gold standard for pesticide analysis. In this article recent developments in LC–MS–MS technology to meet increasing regulatory demands and productivity targets in quality control and contract laboratories are explored.
Liquid chromatography coupled to triple quadrupole tandem mass spectrometry (LC–MS–MS) in multiple reaction monitoring (MRM) mode is widely considered the gold standard for pesticide analysis. However, when screening for either positively or negatively charged ions, two methods have to be run, in addition to sample preparation and method development steps, which can lead to lengthy analysis times. In this article recent developments in LC–MS–MS technology to meet increasing regulatory demands and productivity targets in quality control (QC) and contract laboratories are explored.
By 2050, the United Nations predict that the population of the world will have grown to approximately 8.1 billion.1 This means that between 1950 and 2050 the population of the planet will have more than doubled. Agrochemicals to enhance crop yield are now widely accepted to be important in the development of food production strategies to sustain this exponential growth. The impact of increased reliance on agrochemicals is already evident — in 2011 the US Food and Drug Association (US FDA) screened for 500 pesticide residues, an increase of 38 on the previous year, or 7.6%.2 Increasing crop export trade between regions with differing pesticide control regulations is further fuelling the need for screening. However, many commercial and quality control (QC) laboratories are struggling to improve routine analysis productivity without affecting method robustness or reliability of results. Therefore, reducing analysis time is crucial for food testing laboratories to meet increased demand in a safe and cost-effective manner.

Photo Credit: Foodcollection RF/Getty Images
Liquid chromatography coupled with tandem mass spectrometry (LC–MS–MS) in multiple reaction monitoring (MRM) mode is used widely for pesticide analysis. Triple quadrupole MS enables quantification of hundreds of analytes across a wide mass range with high sensitivity, linearity, and accuracy. However, conventional LC–MS–MS techniques have several limitations in terms of efficiency. One of the most common limitations is that analysis must be performed separately in both positive and negative modes to detect oppositely charged ions, doubling the sample used and the analysis time. A further limitation is the time spent on sample preparation; a routine pesticide screening method can potentially cover hundreds of distinct MRM transitions.
To overcome these limitations, a single detection procedure that simultaneously switches between detecting positive and negative ions can potentially halve analysis times. High performance LC–MS–MS techniques combine single method analysis with minimal method development and straightforward "dilute-and-shoot" sample preparation to increase laboratory throughput.
Accelerating Analysis
When performing LC–MS–MS in MRM mode, compounds are ionized into either cationic (positive) or anionic (negative) ions, often using electrospray ionization (ESI). The resulting ions are then fragmented within a collision cell to produce multiple fragment ions indicative of the original pesticide compound. Targeting these multiple transitions ensures high selectivity and specificity for robust analysis. It is for this reason that triple quadrupole MS with MRM is recommended by regulatory agencies worldwide as the base of effective pesticide screening methods.3 Conventional MRM methods screen for one group of ions at a time. To account for oppositely charged species, such as positively charged pesticide cyazofamid and negatively ionized fipronil, a second screening procedure is required.
Developing a single analysis method requires simultaneous switching between negative/positive detection where appropriate. This is further complicated for cyazofamid and fipronil, because the compounds co-elute with the same retention time. However, modern instruments can compute and assign scan-time for hundreds of multi-residue pesticide assays and automatically switch between positive and negative modes where appropriate. Figure 1 shows a screenshot of a timed MRM window for 250 pesticides, where rapid polarity switching enables positive and negative compound analysis within the same method, accelerating analysis time and simplifying method development.

Figure 1: Compound-based scanning (CBS) timed MRM window for 250 pesticides allows rapid polarity switching to detect positive and negative compounds.
The next step to optimize the analysis is to reduce sample preparation. Eliminating the signal from complex food matrices is essential to sensitivity and selectivity. The "dilute-and-shoot" method of sample preparation overcomes this by heavily diluting the sample, often 100 to 200 times over, to minimize matrix effects. Dilute-and-shoot is widely preferred to alternative sample preparation methods, such as solid-phase extraction (SPE), because of its operational simplicity. However, many techniques are unable to cope with detecting trace analytes at these extremely low levels. In recent years, developments in LC–MS–MS have focused on improving analysis sensitivity by making dilute-and-shoot a reliable method for detecting components down to sub-ppm levels. Positive-negative switching and easy sample preparation together reduce both the duty and analytical cycle for high throughput pesticide quantification.
Case Study: Detection of Pesticides in Fruit and Vegetable juice
Screening of apple, cranberry, orange, and vegetable juice samples was performed using positive-negative ion switching with "dilute-and-shoot" sample preparation to detect for the presence of 250 pesticides.4
Method: Samples of juice (50 μL) were diluted with 450 μL of solvent (methanol/water (10/90, v/v) in the filter vial (Part number 85531-5, Thomson Instrument Company) using a press filter plunger (0.2-μm PVDF) to filter. Samples were separated using ultrahigh-pressure liquid chromatography (UHPLC) and analyzed using an EVOQ Elite LC–MS–MS (Bruker) with a CTC autosampler for automated loading.
LC Parameters: Column: 150 mm × 3 mm, 3-μm YMC-Pack ODS-AQ; column temperature: 40 °C; injection volume: 30 μL; mobile phase A: 5 mM ammonium fluoride in water; mobile phase B: methanol
MS Parameters: Source: HESI spray voltage (positive): 4000 V spray voltage (negative) 4000 V; cone gas flow: 20 units; cone temperature: 250 °C; heated probe gas flow: 40 units; heated probe temperature: 400 °C; nebulizer gas flow: 60 units; exhaust gas: On.
Results: Compound-based scanning (CBS) automatically assigned dwell times for MRM detection based on the peak width and data points required, enabling polarity switching of 250 positive and negative compounds for over 500 MRM transitions. Figure 2 shows the total ion chromatogram for the five samples. Figure 3 shows the calibration curve for fipronil and cyazofamid and their co-eluting chromatographic plots. The calibration curve with triplicate injections showed excellent linearity, between 0.01 ppb and 10 ppb, and R2 of 0.999 for both pesticides. The response factor (RSD) over three orders of range was <5%, indicating good reproducibility.

Figure 2: Total ion chromatograms of (from top to bottom) apple juice, orange juice, cranberry juice, grape juice, and vegetable juice.
Overall a total of 12 pesticides were detected in the apple, orange, cranberry, or vegetable juice samples (Table 1). No pesticides were detected in the white grape juice. The presence of multiple pesticides suggests that the juices may come from multiple sources of raw materials, or from pooled juices.
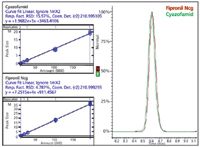
Figure 3: Calibration curves for negative pesticide fipronil (top left) and positive pesticide cyazofamid (bottom left).
The entire analysis was achieved in 18 min. Excellent sensitivity and linearity within the sub-ppm region indicated that dilute-and-shoot is an effective preparation method, even for trace pesticide residues.
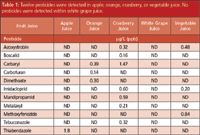
Table 1: Twelve pesticides were detected in apple, orange, cranberry, or vegetable juice. No pesticides were detected within white grape juice.
Summary
Few would deny the importance of pesticides in crop growth, or contest their significance in feeding an ever-growing global population. Enforcing the rigorous regulations that underpin pesticide use is of the highest priority to ensuring consumer safety. Advanced LC–MS–MS techniques provide a platform for the fast and sensitive quantification of hundreds of pesticides using a single positive-negative switching method. Simplified sample preparation and easy method development allows food analyzers to enforce regulatory demands, improve the efficiency of high throughput detection, and ensure the highest level of sensitivity and accuracy, even for trace compound analysis within complex matrices.
References
1. http://www.un.org/esa/population/publications/longrange2/WorldPop2300final.pdf
2. Pesticide Monitoring Programme — 2010 US Food and Drug Administration pesticide report http://www.fda.gov/downloads/Food/FoodborneIllnessContaminants/Pesticides/UCM382443.pdf
3. QuEChERS food testing protocol AOAC 2007.01 EN15662.
4. Screening and Quantitation of 250 Pesticides in Fruit Juices with Positive/Negative Switching LC–MS–MS — Bruker Chemical and Applied Markets poster
Joe Anacleto is the VP for Bruker Daltonics Applied Markets Business Group.
Zicheng Yang is a senior field application specialist at Bruker Daltonics.
Louis Maljers is a field applications manager at Bruker Daltonics.
E-mail: cam-sales@bruker.com
Website: www.bruker.com
This article is from The Column. The full issue can be found here:http://images2.advanstar.com/PixelMags/lctc/digitaledition/October06-2014-uk.html#2

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.












