Portable FT-IR and Raman Spectroscopy for Explosives Identification
Special Issues
This article discusses instruments that can be used in the field to rapidly and accurately identify various explosives and their precursors.
Explosives ordnance disposal (EOD) specialists, bomb technicians, forensic scientists, hazardous material (HazMat) technicians, and first responders working in the realm of CBRNE (chemical, biological, radiological, nuclear, and explosives) meet a broad set of challenges in the course of their missions, and consequently require a broad set of tools to assist them in the completion of their duties. Two especially useful technologies in the battle against improvised explosive devices (IEDs) are Raman spectroscopy and Fourier transform–infrared spectroscopy (FT-IR). Recent advances in miniaturization, optical technologies, software development, and packaging have changed the landscape of laboratory-quality analytical devices for explosive identification and put the knowledge — and the instruments — literally in the hands of the first responder who needs them most.
Tools in the Fight Against Improvised Explosive Devices
IEDs are gaining traction as the weapon of choice for many terrorist organizations worldwide, due in part to the relative ease of production and widespread availability of raw materials. Bombings were responsible for more than half the fatalities and 85% of terrorism-related injuries in 2006 alone. The United States Department of Defense (DoD) spent nearly $4.0 billion in 2008 for the development and fielding of measures to defeat IEDs.
A key strategy to stop IED production is to locate the toxic industrial chemicals (TICs) and toxic industrial materials (TIMs) that serve as precursors as they are transported through checkpoints. TICs and TIMs are common industrial products that can be mixed, dissolved, blended, or disguised to obscure their true identity. IEDs are not fueled by traditional familiar explosive materials such as TNT, but are made in crude chemical laboratories using industrial chemicals and common household items. One method to stop the trafficking of these precursor materials is to use handheld chemical identification instrumentation in the field at checkpoints and borders. Security coalition partners and law enforcement personnel have demanded accurate, rugged, and portable tools to aid them in the fight against the growing global IED threat.
Safety and security personnel with a need for field-deployable analytical instruments include the military and civilian first responders responsible for evaluating and countering chemical, natural, and biological threats. These individuals work for U.S. and foreign government agencies as well as state and local agencies, such as fire departments, law enforcement agencies, and forensic laboratories.
Coalition partners and civilian agencies are working quickly to develop systems to counter the IED threat, equipping the first responders with the right tools to protect themselves and the community. This includes new detection technologies, training, and forensics to quickly identify the threat, find illicit labs, and disable insurgents' capabilities.
A standard set of instruments used by first responders includes trace detection (vapor and particulates), gas, biological, radiological, nuclear, and chemical identification (Figure 1). The focus of this article centers on solid and liquid chemical identification and recent innovations and advances in this area.

Figure 1: Common tools in a first responders toolbox.
In the safety and security market, a fundamental need exists for analytical instruments that can identify unknown chemical substances quickly and precisely at the point of use so that the appropriate course of action can be taken. For these applications, response time and accuracy are critical to save lives, prevent major economic and public disruptions, and aid in resolving emergencies. Instruments located at remote laboratories, while accurate, do not adequately meet the need for a timely response. Portable instruments are sought after to quickly verify the contents of tankers, drums, bags, and bottles at checkpoints.
First-generation portable chemical identification solutions have been developed during the past 10 years to address the growing need for onsite analysis and have primarily used FT-IR spectroscopy for field identification of dangerous chemical and biological substances. While more suitable than laboratory instruments for these onsite applications, first-generation portable products have some important limitations. Although portable, these instruments are still too large to be handheld, they are not certified for use in the hazard zone, and they require a sample of the unknown substance to be collected by first responders and brought to the instrument. When dealing with an unknown, first responders avoid touching or transporting the substance as much as possible because this creates additional safety risks for the responder and the community.
Raman and FT-IR spectroscopy are ideal technologies for the accurate identification of liquid and solid TICs and TIMs, as well as numerous explosives and their precursors.
Molecular Spectroscopy
Analytical instruments are used to detect, identify, authenticate, and quantify chemical and biological substances. One key form of analytical instruments utilizes molecular spectroscopy.
Molecular spectroscopy involves projecting electromagnetic energy onto a substance and analyzing the resulting interaction. The two major classes of molecular spectroscopy are nuclear magnetic resonance (NMR) and optical. NMR involves directing radio waves at a substance while subjecting it to a magnetic field and measuring the response. Located in laboratories and medical institutions, instruments using NMR are generally large, high-cost instruments used to detect and quantify trace substances.
On the other hand, optical instruments fall into four categories: ultraviolet–visible (UV–vis), near-infrared (NIR), mid-infrared (MIR), and Raman. Whereas NMR employs radio waves, optical spectroscopy employs different types of energy or light waves. Two forms of molecular spectroscopy well-suited for the identification of solid or liquid explosives are Raman and FT-IR.
Both Raman spectroscopy and FT-IR spectroscopy measure the interaction of energy with the molecular bonds in a sample of an unknown liquid or solid material. FT-IR measures how much light is absorbed by the bonds of a vibrating molecule (or the remaining energy from the original light source that can be measured after being passed through the substance). In comparison, Raman measures the energy that is emitted or scattered after being excited by a laser. Both techniques are illustrated in Figure 2.

Figure 2: Comparison of how spectra are measured by FT-IR and Raman spectroscopy technologies.
FT-IR and Raman spectroscopy are the most precise of the optical techniques, and each offers distinct advantages in specific applications. However, these techniques are distinctly different but complementary methods of spectral analysis. The response of an unknown substance to each technique is dictated by the substance's unique molecular structure, with certain substances responding extremely well to FT-IR and others responding better to Raman spectroscopy. Used together, FT-IR and Raman spectroscopy provide a broader range of unknown substance identification — and better protection for the responder and the community. Figure 3 shows the complementary nature of Raman and FT-IR spectroscopy techniques that are explored in subsequent sections.
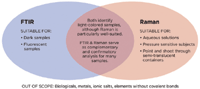
Figure 3: Complementary capabilities of FT-IR and Raman spectroscopy.
Raman Spectroscopy for Explosive Identification
Raman is a vibrational spectroscopy technique in which a single-wavelength laser is directed toward a sample. The laser energy, which excites the bonds of the molecule, generates or "scatters" measurable light and can be used to identify the material in question. Raman is highly effective at extracting a reliable and accurate identification of an unknown substance based on the underlying chemistry of an unknown substance. Raman is best used in the following scenarios:
- Sealed containers. Raman spectroscopy enables users to collect data through semitransparent containers for both solids and liquids without disturbing the sample.
- Aqueous solutions. Water has a very weak Raman signal, which allows Raman instrumentation to virtually disregard water in samples and concentrate on potentially more threatening materials. This makes samples diluted in water easily identifiable with Raman.
- White or light-colored powders. White powders with nonpolar covalent chemical bonds have very strong Raman signals. Handheld Raman instruments (such as Ahura Scientific's FirstDefender) make onsite rapid explosive identification a reality. Users are able to quickly and positively identify unknown liquids, solids, and mixtures — usually in less than 30 s. Using a laser to interrogate the sample and a sensitive detector to measure the scattered light, a Raman spectrum is created with a wavelength and intensity that is specific to the sample molecule.
Instruments collect the molecular spectrum of the substance in question, and onboard chemometric algorithms subsequently identify the chemical composition of the material by matching the acquired spectrum against those stored in the instrument. Some instruments are further able to identify explosive precursors in mixtures.
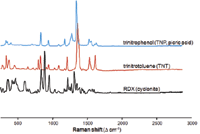
Figure 4: Unique Raman spectra of TNP, TNT, and RDX.
Figure 4 shows the Raman spectra of the explosive threats TNP, TNT, and cyclonite (commonly referred to as RDX). Each material can be uniquely identified based on its Raman spectrum. Another example of Raman explosive spectra is illustrated in Figure 5.
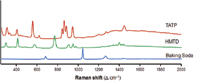
Figure 5: Raman spectra of TATP, HMTD, and baking soda.
FT-IR Spectroscopy for Explosive Identification
FT-IR is an absorption spectroscopy technique in which an infrared light is passed through the sample. Some wavelengths may be absorbed, while others merely pass through the sample unaffected. Specific molecular bonds absorb a specific amount of energy, and these losses of energy correspond to the peaks returned during an analysis.
FT-IR absorptions are strong and provide outstanding and easily interpretable results for substances that contain polar covalent bonds. FT-IR is best used as a potential explosive identification technique in the following scenarios:
- Fluorescent materials. FT-IR is ideal for fluorescent samples.
An illustration of collected FT-IR spectra for Semtex 1H, Detasheet, and black powder can be seen in Figure 6. The sharp peaks make for clear and easily interpretable results.
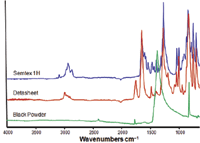
Figure 6: FT-IR spectra for the explosive materials Semtex 1H, Detasheet, and black powder.
In the Real World . . . Mixtures, Precursors, Solvents, and Impurities
Obtaining accurate and actionable information is what first responders and bomb technicians value most.
Traditional laboratory-grade spectroscopy software commonly employs library searching methods in which a multi-wavelength measure of similarity such as correlation or Euclidean distance is used to rank library items. From a long list of ranked library items, an expert user can more quickly compare "hits" against the unknown and make a final assessment.
The primary difficulty for field use by non-experts is that the measures of similarity that are most commonly used as a hit-quality index have no probabilistic correspondence. A correlation of 0.96 certainly does not mean a listed library item is 96% likely. To convert any such similarity index into a probabilistic measure would require a definition for both the expectation and the sampling distribution of the statistic, neither of which can be analytically defined for traditional similarity metrics such as correlation or Euclidean distance in spectroscopy. Thus, field use of traditional library search software is hampered by very high "false-identification" rates.
The embedded analytics in contemporary chemical identification instruments create probabilistic models from their onboard library. The devices are situationally and environmentally aware so that through analytic modeling of the device performance characteristics, probabilistic models are constructed in real time and real probabilities for the basis for the results. The devices directly test for statistical equivalence between the measured material and each library material. The finesse of the calculations results in extremely low false-identification rates (1 in 10,000 [1]) with strong positive identification. These capabilities allow the instruments to present meaningful information that can be acted upon without extensive chemical knowledge.
Conclusion
Rapid, precise identification of explosives is one of the key tasks for homeland security and public safety personnel, especially with the marked increase of IED usage worldwide. Portable X-ray explosive detection and trained canines add critical capabilities for first responders; however, additional tools are also available to further assist with identification of unknown materials. Instruments that can be used in the field to rapidly and accurately identify various explosives — and their precursors — are essential tools for first responders responsible for the safety and security of the community. Two complementary response techniques for the identification of unknown solid or liquid chemicals are Raman and FT-IR spectroscopy. Each technique offers distinct advantages in specific explosive applications, and when used together, they extend the capabilities of the first responder's toolkit and provide rapid and accurate results on which a responder can rely, enabling more rapid incident response times.
Duane Sword is Vice President, Product Management and Marketing, Ahura Scientific, Inc., Wilmington, MA.
References
(1) C.D. Brown and R.L. Green, "Performance Characterization of Material Identification Systems." SPIE Proceedings Optics East 2006, Boston.


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









