Per- and Polyfluoroalkyl Substances (PFAS) or Interference? Using High-Resolution Mass Spectrometry as an Investigative Tool in Food Analysis
Accurate quantification of per- and polyfluoroalkyl substances (PFAS) in food is necessary to understand potential dietary exposure. While beneficial for most applications, quantification of PFAS using low-resolution triple quadrupole instruments can be complicated by the presence of co-eluted interferences, which can result in false positives or inaccurate PFAS concentrations. This article discusses the use of high-resolution mass spectrometry (HRMS) to distinguish between matrix interferences and PFAS compounds in a variety of food matrices, and incorporation of these interferences into routine triple quadrupole methods for monitoring.
Due to the extensive use of per- and polyfluoroalkyl substances (PFAS) in consumer products and industrial applications since the 1950s, these compounds have been detected in the environment, humans, and food. Therefore, the accurate quantification and determination of PFAS in foods is important to better understand potential dietary exposure.
Since the identification of taurodeoxycholic acids as an interference with perfluorooctane sulfonic acid (PFOS) in human serum (1), interferences continue to be identified with individual PFAS in samples including environmental, biological, and food matrices. As analytical methods continue to expand with additional analytes and matrices, the detection of interferences and false positives has also increased.
Routine low-resolution triple quadrupole analysis continues to be the primary analytical technique for the quantification of PFAS in food and environmental samples due to its lower cost, ease of use, and typically lower detection limits compared to high-resolution mass spectrometry (HRMS). However, the unit mass resolution of triple quadrupole methods is insufficient to differentiate PFAS compounds from potential interferences within ± 1 Dalton (Da) of the target precursor mass and tandem mass spectrometry (MS/MS) transition mass. This can lead to false positives or overestimation of PFAS concentrations. This is particularly challenging for complex matrices, such as foods, and for short-chain PFAS compounds with only one transition ion, such as perfluorobutanoic acid (PFBA) and perfluoropentanoic acid (PFPeA). This challenge is exacerbated when the only available transition is not unique or diagnostic (for example, loss of CO2 from a compound containing a carboxyl group). These shorter chain analytes are known to uptake into plants and can transport to the leafy portions (2), which makes their accurate quantification in plant and agricultural products challenging. In addition, recent publications have identified PFPeA interferences in chocolate-containing foods and shellfish (3,4), and PFBA interferences from fatty acids in biological and environmental matrices (4,5).
High-resolution mass spectrometry (HRMS), often using orbital ion trap or time-of-flight (TOF) instruments, can be used to differentiate PFAS compounds from matrix interferents that differ in mass by ± 0.0001 to 0.0010 Da (less than a ±10 ppm mass difference). Therefore, PFAS analytes, such as PFBA and PFPeA, can be identified using the accurate mass of the precursor and product ions for PFBA (m/z 212.9792 to m/z 168.9894) and PFPeA (m/z 262.9760 to m/z 218.9862). For this article, due to the order of magnitude difference in mass accuracy between instruments, Da and ppm will be used to describe the mass accuracy of triple quadrupole and HRMS instruments, respectively.
This article describes an approach that can be used to differentiate matrix interferences from true positive PFAS detections using HRMS and tandem mass spectrometry (MS/ MS). The approach is demonstrated using a matrix interferent for PFPeA that is commonly found in chocolate; however, this procedure can be broadly applied to diverse matrices and other PFAS compounds with suspected interferents.
Determination of Matrix Interferences Using HRMS
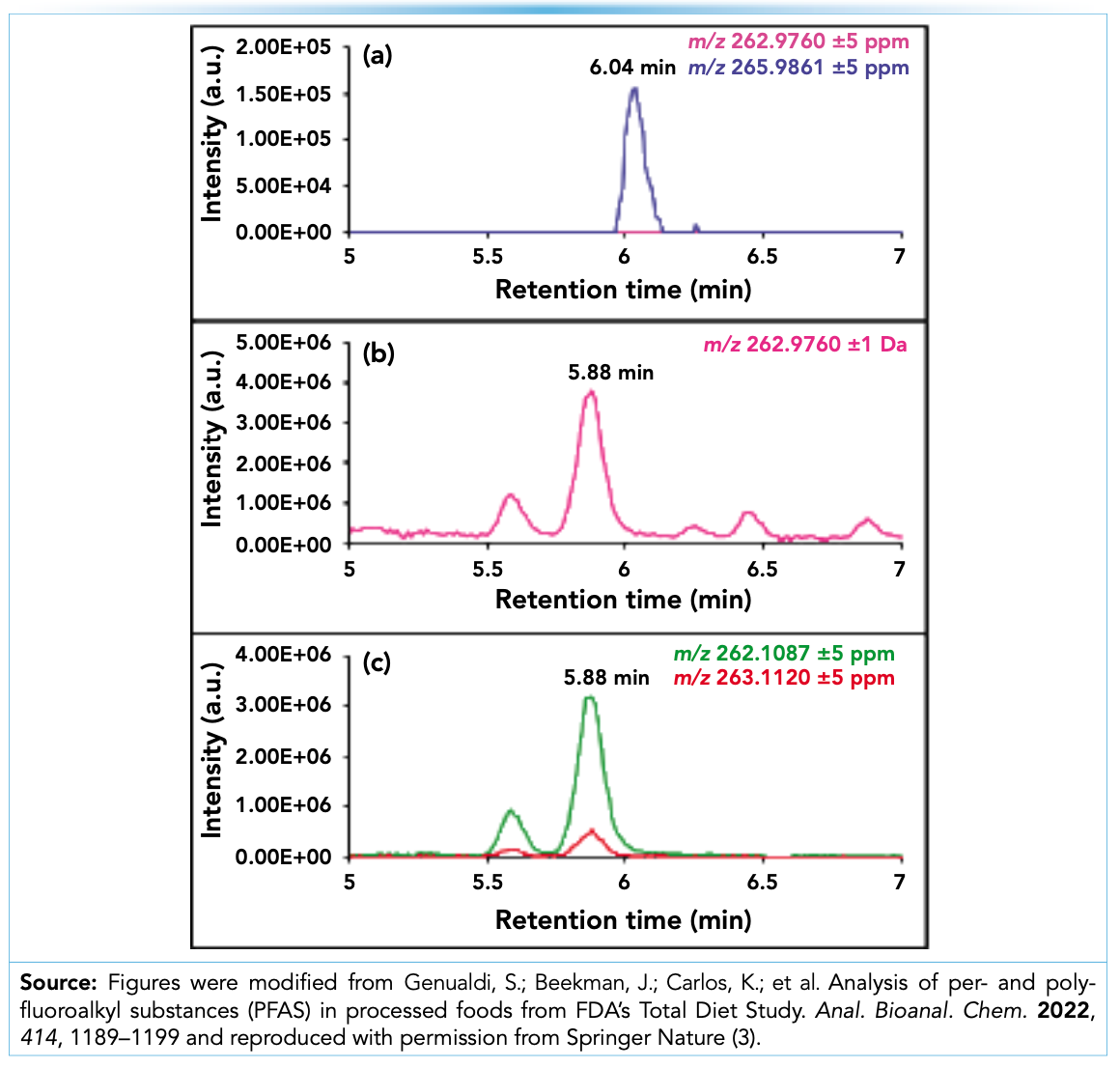
When there is a suspected matrix interference based on the triple quadrupole data (such as positive detections for PFBA and PFPeA, retention time shifts relative to corresponding labeled internal standards, or unexpectedly high concentrations), the sample can be analyzed using liquid chromatography (LC)–HRMS with the same or a similar LC method as used for the triple quadrupole method. To illustrate the process for accurately identifying interferents with HRMS, an example is shown from a chocolate chip cookie with a suspected PFBA interferent analyzed with both triple quadrupole (Sciex QTRAP 6500+ system) and HRMS (Thermo Q-Exactive Orbitrap) instrumentation. The full scan mass spectral data is examined first by generating extracted ion chromatograms (EICs) for the accurate masses of the suspected PFAS compounds and any relevant surrogate compounds with a mass range of ±1 Da to mimic the triple quadrupole mass accuracy and ±5 ppm to utilize accurate mass. Figure 1a shows the EICs for PFPeA (pink; m/z 262.9760 ± 5 ppm) and the labeled PFPeA surrogate (blue; m/z 265.9861 ± 5 ppm); the latter was spiked into the chocolate chip cookie sample. There is no peak for PFPeA at 6.04 min (as expected based on the labeled PFPeA retention time). Figure 1b shows an EIC for a mass range of ±1 Da of PFPeA (m/z 261.9760 to m/z 263.9760) which can be used to directly compare the HRMS results to the triple quadrupole data. The data show that the peak at 5.88 min is likely a matrix interferent which caused a false positive detection on the triple quadrupole instrument due to the retention time proximity to the standard (shown in Figures 1a and 1b). The accurate mass of the suspected interferent at 5.88 min was determined to be m/z 263.1120. Interestingly, this is actually the 13C isotope of m/z 262.1087. Figure 1c shows the extracted ion chromatograms (EICs) for the exact masses of the 12C (m/z 262.1087 ± 5 ppm) and 13C (m/z 263.1120 ± 5 ppm) isotopes, where the elution profiles align well with each other and the EIC of the suspected triple quadrupole interferent in Figure 1b.
FIGURE 1: LC–HRMS EICs generated from a chocolate chip cookie sample for (a) PFPeA (pink; m/z 262.9760 ± 5 ppm) and the labeled PFPeA surrogate (blue; m/z 265.9861 ± 5 ppm); (b) PFPeA m/z 262.9760 ± 1 Da; and (c) the suspected interferent at m/z 262.1087 ± 5 ppm (green) and its associated 13C isotope (red; m/z 263.1120 ± 5 ppm).
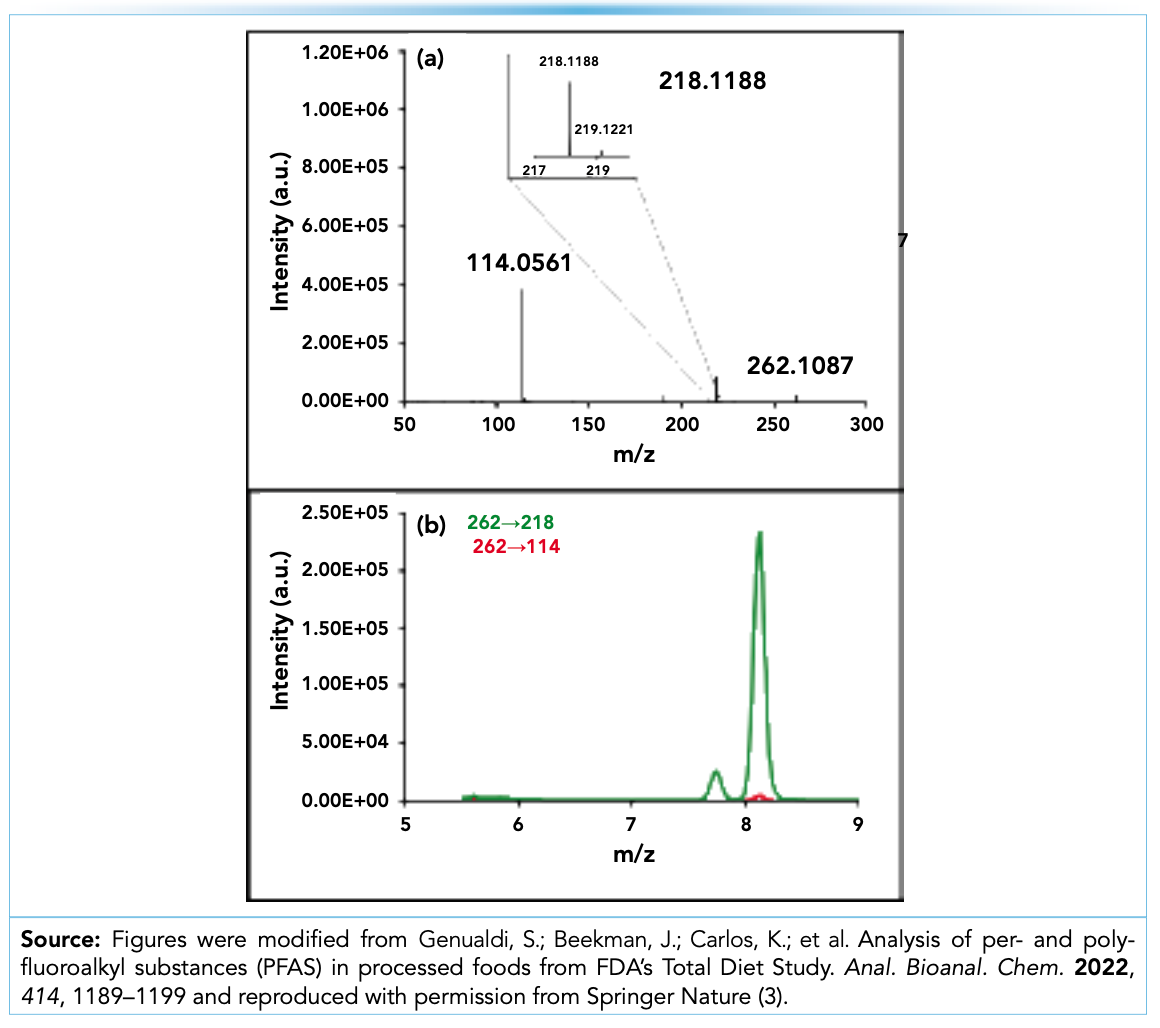
Once the mass-to-charge of the suspected interferent is found, MS/MS data is examined to verify that the interferent precursor generates a product ion within ±1 Da of the expected transition mass (for example, m/z 262.9760 → m/z 218.9862 for PFPeA) in the MS/MS spectrum. For example, Figure 2a shows the MS/MS spectrum for the suspected PFPeA interferent at m/z 262.1087 in the cookie. The most abundant fragment in the MS/MS spectrum is m/z 218.1188, which has a 13C isotope at m/z 219.1221. This fragment is within the ±1 Da window of the PFPeA fragment mass at m/z 218.9862, further indicating this compound is likely the false positive being detected on the triple quadrupole instrument.
FIGURE 2: (a) The HRMS-MS/MS spectrum for the suspected interferent at m/z 262.1087; and (b) EICs for the m/z 262 to m/z 218 (green) and m/z 262 to m/z 114 (red) transitions of the suspected interference on the triple quadrupole instrument.
At this point, attempts can be made to identify the interferent. If the structure of the interferent is known, additional sample preparation steps can be developed to remove it. As mentioned previously, taurodeoxycholic acid has been identified as a common component in biological and food matrices which interferes with the main transition used to quantify PFOS (m/z 499 → m/z 80). This compound can be removed from samples with an additional cleanup step utilizing graphitized black carbon during sample preparation (6). However, identifying interferents and developing additional sample preparation procedures for each can require significant time and resources, which may not be feasible in every case. Because interferences will continue to arise as more PFAS compounds are monitored and additional foods are tested, there is a need for a rapid approach to limit these interferences.
One way to address this challenge is by leveraging additional product ions in the MS/MS spectrum of the interferent generated on the HRMS instrument. In the chocolate chip cookie example, the suspected interferent generated a product ion of m/z 114.0561 as shown in Figure 2a. Because this product ion is not present for PFPeA, the m/z 262 to m/z 114 transition can be added to the triple quadrupole method to confirm the presence of this interference (Figure 2b). This interferent was also confirmed by Bangma and co-authors in their investigation of PFPeA in cocoa mix (4). As more interferents are identified and documented, these additional transitions can be added to triple quadrupole methods to rapidly distinguish them from PFAS compounds in the same analysis, without the need for additional sample preparation development/procedures. This is also extremely valuable for labs that may not have access to HRMS instrumentation.
Conclusion
The approach described here is broadly applicable to diverse PFAS compounds and matrices, making it amenable to the rapid pace at which new PFAS interferences are being found. In addition to the PFPeA in the chocolate chip cookie example, this approach has been successfully applied to distinguish interferences from PFBA in deer meat samples and from 4:2 fluorotelomer sulfonic acid (4:2 FTS) in corn snaplage using a different HRMS instrument (Sciex ZenoTOF 7600 system). Given the challenges associated with interferences in complex matrices, the use of HRMS is a powerful tool for PFAS confirmation and for investigation of interferences.
HRMS is capable of distinguishing matrix interferents from targeted PFAS compounds in complex matrices based on mass differences within ± 0.0001–0.0010 Da (for example, ±5 to 10 ppm), reducing the risk of false positives and overestimation. In addition, the full MS/MS spectrum of interferents can be leveraged to establish alternate transitions to distinguish PFAS and interferent compounds in the same analytical data acquisition on triple quadrupole mass spectrometers, without requiring interferent identification and additional sample preparation procedures. This approach ensures that PFAS amounts reported in foods are accurate when used for the calculation of dietary exposure estimates.
References
(1) Benskin, J. P.; Bataineh, M.; Martin, J. W. Simultaneous Characterization of Perfluoroalkyl Carboxylate, Sulfonate, and Sulfonamide Isomers by Liquid Chromatography−Tandem Mass Spectrometry. Anal. Chem. 2007, 79, 6455–6464. DOI: 10.1021/ac070802d
(2) Blaine, A. C.; Rich, C. D.; Hundal, L. S.; et al. Uptake of Perfluoroalkyl Acids into Edible Crops via Land Applied Biosolids: Field and Greenhouse Studies. Environ. Sci. Tech. 2013, 47, 14062–14069. DOI: 10.1021/es403094q
(3) Genualdi, S.; Beekman, J.; Carlos, K.; et al. Analysis of Per- and Poly-fluoroalkyl Substances (PFAS) in Processed Foods from FDA’s Total Diet Study. Anal. Bioanal. Chem. 2022, 414, 1189–1199. DOI: 10.1007/s00216-021-03610-2
(4) Bangma, J.; McCord, J.; Giffard, N.; et al. Analytical Method Interferences for Perfluoropentanoic Acid (PFPeA) and Perfluorobutanoic Acid (PFBA) in Biological and Environmental Samples. Chemosphere 2023, 315, 137722. DOI: 10.1016/j.chemosphere.2022.137722
(5) Bangma, J. T.; Reiner, J.; Fry, R. C.; et al. Identification of an Analytical Method Interference for Perfluorobutanoic Acid in Biological Samples. Environ. Sci. Tech. Letters 2021, 8, 1085–1090. DOI: 10.1021/acs.estlett.1c00901
(6) Jack, R. The Evolving Developments in Sample Preparation for PFAS in Environmental Samples. Column 2022, 18, 15–19.
Brian Ng, Christine M. Fisher, Susan Genualdi, Wendy Young, Elsie Peprah, Ann M. Knolhoff, and Lowri deJager are with the USFDA Center for Food Safety and Applied Nutrition, in College Park, Maryland. Direct correspondence to: Susan.Genualdi@fda.hhs.gov

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.









