New Technologies That Accelerate Persistent Organic Pollutant (POP) Sample Preparation
Despite best efforts in 2004 to ban their use, persistent organic pollutants (POPs) remain prevalent across the globe, including in soil. To protect human health, agricultural and environmental soil require careful investigation, but preparing samples for gas chromatography–mass spectrometry (GC–MS) analysis is time-consuming, and mostly done manually. Accelerated solvent extraction (ASE) has been the preferred preparation method for the past few decades, and while we have seen advancements, the method remains manual. Now, new technology offers parallel sample processing, combined extraction and evaporation, and automation—leading to faster analysis, reduced risk of error, and freed-up time for personnel.
Persistent organic pollutants (POPs) are still a cause of safety concern. After significant efforts from the Stockholm Convention on POPs to reduce and eliminate these compounds in 2004 (1), many are now banned. However, their persistence means they can remain in soil for a long time. From soil, POPs can make their way into the food chain, causing adverse effects. Accurate POP quantitation is therefore essential to protect human health.
Proper sample preparation is critical for easier gas chromatography–mass spectrometry (GC–MS) analysis. Cleaner samples with fewer compounds reduce competitive ionization and enhance the signal, allowing analysis to better meet reproductivity, recovery, and sensitivity requirements. In addition, clean samples greatly reduce instrument maintenance, as dirty samples can foul up equipment and cause ion suppression, leading to signal loss.
To prepare samples for analysis, the compounds need to be removed from solid (or semi-solid) matrices and taken up into a liquid for injection. However, the workflow is complex, consisting of three steps: extraction, cleanup, and evaporation (Figure 1).
FIGURE 1: The POP sample preparation workflow.
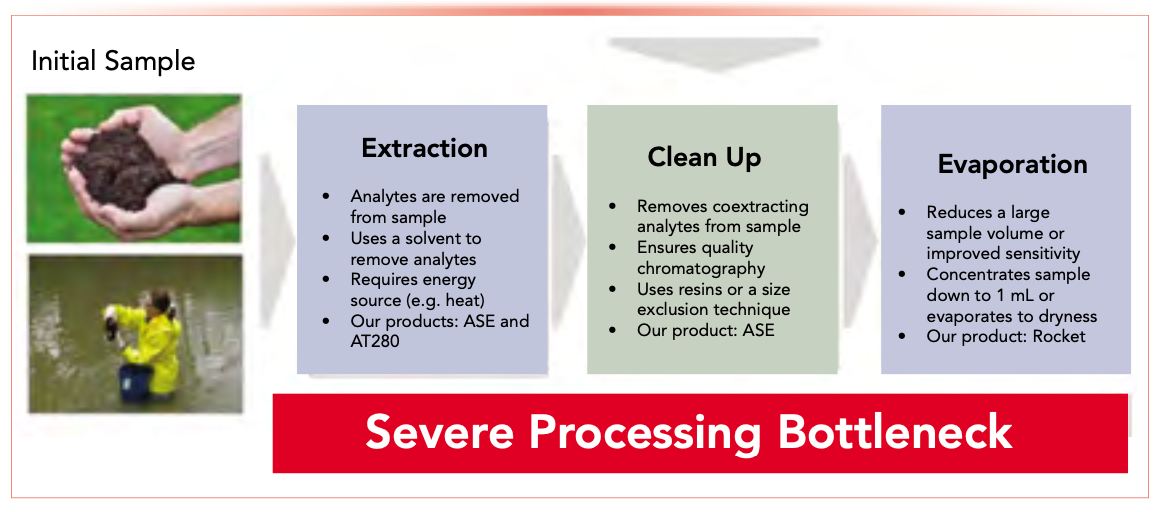
Two-thirds of all processing time is spent preparing the samples, which is still mostly manual. Despite many advances in preparation, it remains resource-intensive, requiring personnel to do laborious and tedious processes. It is also error-prone: over 80% of all laboratory errors occur during sample preparation. These drawbacks have profound influence on the overall workflows:
- Sample throughput is low, and turnaround times are long
- Increased errors drive up costs for re-running samples, and bring risks to accreditation
- Staff retention and recruitment is challenging, contributing to high turnover and limiting productivity
Accelerated solvent extraction (ASE), a high-temperature and high- pressure technique, offers fast and effective sample extraction. Due to its strengths, ASE is often the technique of choice, but relatively few changes have been made over recent years, offering space for improvement. ASE is, for example, a manual process that requires subsequent cleanup and solvent evaporation to concentrate samples for instrumental analysis. Alternatively, manual processes like Soxhlet can be used; however, it uses large volumes of solvent and takes a lot of time, which a chemist must solely dedicate to the process, which drives up costs.
Now, parallel extraction protocols with automated sample preparation exist as alternatives to manual preparation. Additionally, both extraction and evaporation can be combined in an automated system to save time and drive productivity:
- Freed-up time for personnel: Combining tasks increases walkaway time during sample preparation
- Reduced errors: There is less user intervention required, and no spills occur
- Accurate workflows: Automation allows end-point detection to stop the process at the desired level
New systems feature a gas-assisted extraction mode, which saves on both solvent use and extraction time. After collection, the needles can be rinsed to reduce carryover. Evaporation is then performed in the still-sealed vessels—under gentle vacuum, to avoid the loss of semi-volatile compounds—and a nitrogen (N2) stream is blown through the same collection needle. Samples that are less volatile can also be heated to aid evaporation.
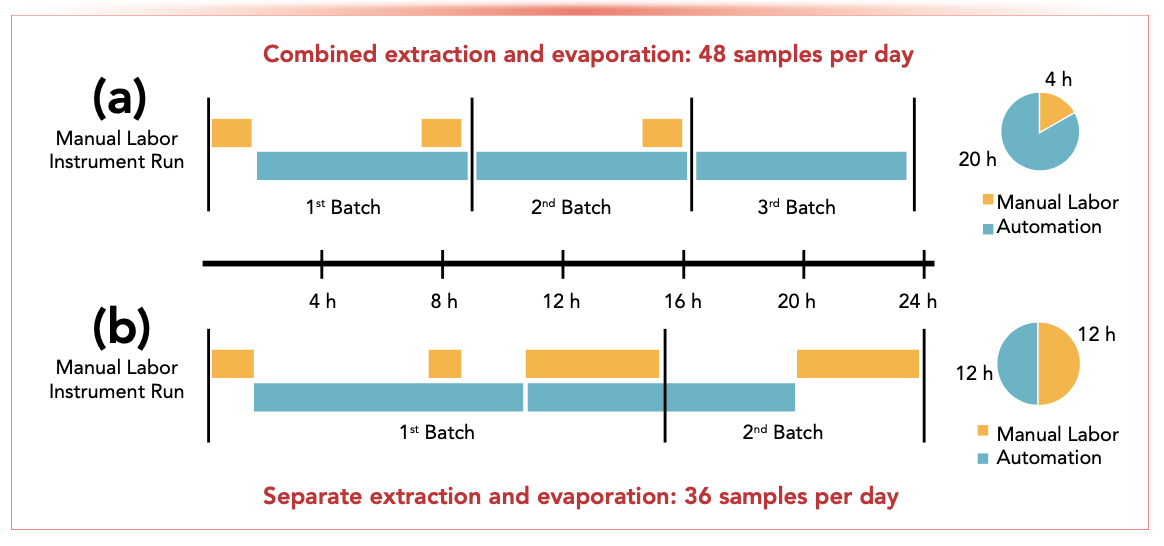
So, how effective are combined and automated extraction and evaporation systems for POP purification (Figure 2)? Here, we explore their suitability for preparing organochlorine pesticides (OCPs), polychlorinated biphenyls (PCBs), and polyaromatic hydrocarbons (PAHs) in soils.
FIGURE 2: Schematic comparing (a) combined, and (b) separate extraction and evaporation systems, showing daily sample throughput and time required for manual labor and automation.
Materials and Methods
OCP Sample Setup
Pesticides and surrogate standards were mixed and diluted with hexane to produce stock solutions. The stock solution was diluted to obtain calibration standards with concentrations of 0.01, 0.02, 0.05, 0.1 and 0.2 μg/mL. Pentachloronitrobenzene (2 μg/mL) was then added to each standard.
PCB Sample Setup
PCB Congener Mix and surrogate standard (2,4,5,6-tetrachloro-m-xylene) were mixed and diluted with hexane to form stock solutions (1 μg/mL).
The stock solution was diluted to make calibration standards of 0.005, 0.02, 0.05, 0.10, 0.20, and 0.3 μg/mL. An internal standard solution of decachlorobiphenyl (10 μg/mL, 20 μL) was then added to each calibration standard.
PAH Sample Setup
PAHs and surrogate standards were mixed and diluted with acetone-methylene chloride in a 1:1 ratio. The resultant stock solutions were diluted to make calibration standards with concentrations of 0.1, 0.2, 0.5, 0.75, 1.0, and 2.0 μg/mL. An internal standard solution of naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12 (20 μg/mL, 20 μL) was then added to each calibration standard.
General Setup
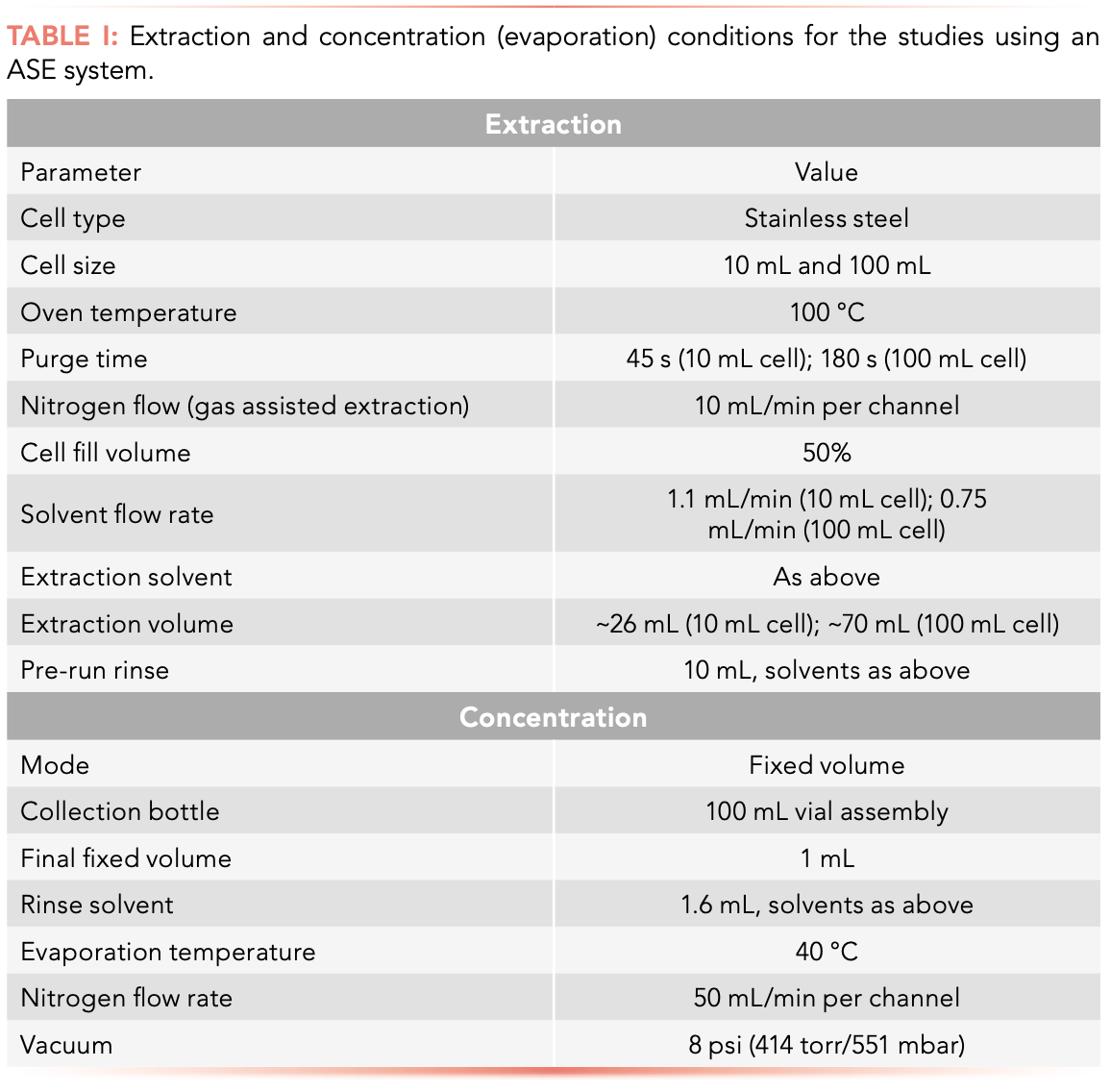
For each study, clean loam soil (2 g) was mixed in a glass beaker with diatomaceous earth (2 g, Dionex ASE Prep DE dispersant). This mixture was poured into an extraction cell and spiked with the appropriate standard, as detailed above. All the samples then underwent ASE using the Thermo Fisher Scientific EXTREVA ASE accelerated solvent extractor, as per the conditions outlined in Table I. The solvents used were acetone-hexane (1:1) for OCPs, hexane for PCBs, and acetone-methylene chloride (1:1) for PAHs.
Results and Discussion
Newer systems combine sample extraction and evaporation in a single automated platform, greatly reducing the need for human intervention. Extraction and concentration of multiple samples is performed in parallel, increasing throughput. As an added bonus, these systems also reduce the volume of solvent used for extraction, making the workflow more cost-effective and environmentally friendly.
OCP Analysis
OCPs, synthetic chlorinated hydrocarbon derivates, were once widely used to protect crops, livestock, buildings, and households from insect damage. But OCPs persist, bioaccumulate, and biomagnify, leading to their restriction and banning in many locations globally, including the United States and the European Union. In addition to their harmful properties, OCPs can be analytically challenging; they are semi-volatile, meaning samples must be carefully cleaned for accurate quantitation.
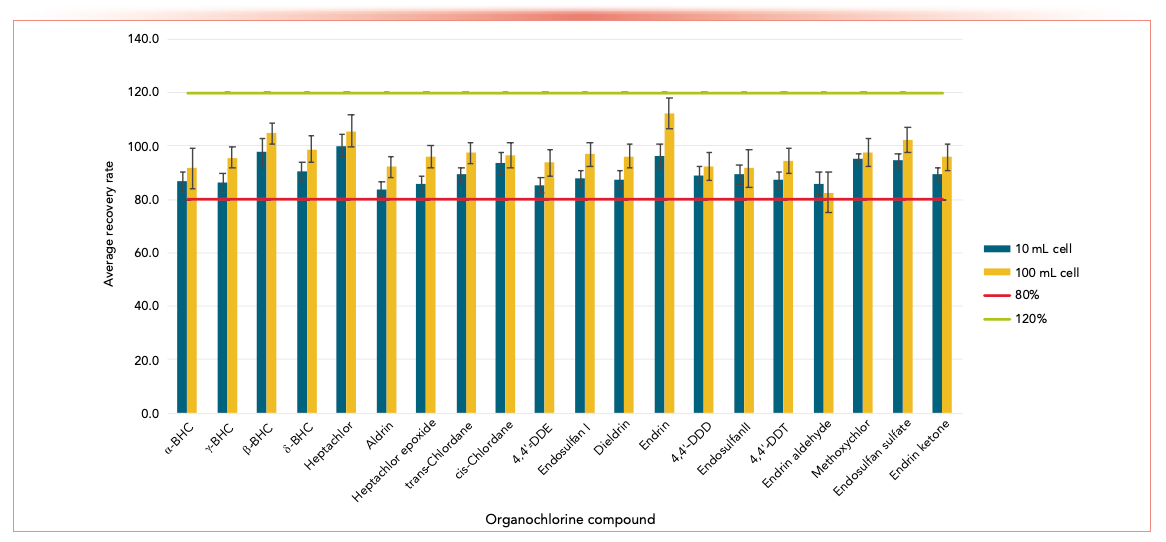
A recent study examined 20 different OCP analytes in spiked soil matrices (2). Compared to single extraction instruments and serial processing, an automated instrument combining extraction and evaporation gave higher throughput for OCP samples, especially in conjunction with parallel processing. Recovery studies were performed, with all OCP recoveries falling between 80% and 115% for both 10 mL and 100 mL cells (Figure 3), meeting the recommended acceptance criteria from the U.S. Environmental Protection Agency (EPA).
FIGURE 3: Average recovery rates of OCP analytes for the 25 μg/kg spike level, with the 80% and 120% limits shown in red and green, respectively.
Further analysis and studies demonstrated the strength of automated and combined extraction and evaporation. Significantly, the relative standard deviation (RSD) was below 7.9% for all standards, demonstrating reproducibility of the method using the system. Carryover studies were also performed—even from high spike samples, very little carryover was detected (0.00–0.43%), ensuring result accuracy and integrity.
PCB Analysis
PCBs are a class of chlorinated hydrocarbons that were widely produced and used until their manufacture was banned in 1979. These resilient, non-flammable, and stable compounds also benefit from high boiling points, making them suitable for a plethora of applications from hydraulic equipment to paints. But PCBs also bioaccumulate and biomagnify, and, given their toxicity, pose a significant threat to human health.
Aiming to simplify PCB analysis, a study used an automated protocol combining extraction and evaporation for sample preparation (3). PCB analytes in soil samples were quantified, with two sample sizes tested: 2 g of soil in a 10 mL cell, and 20 g of soil in a 100 mL cell.
PCB recoveries were between 77.0% and 100.9%, also meeting EPA acceptance criteria (5). Notably, even highly volatile compounds such as PCB18 were extracted with high efficiency. The RSD was also determined and found to be below 20% for all compounds, demonstrating that the extraction and evaporation protocol showed good reproducibility (Table II). Further, proficiency testing (PT) samples were purchased and run, with all PCBs detected within PT published acceptance ranges.
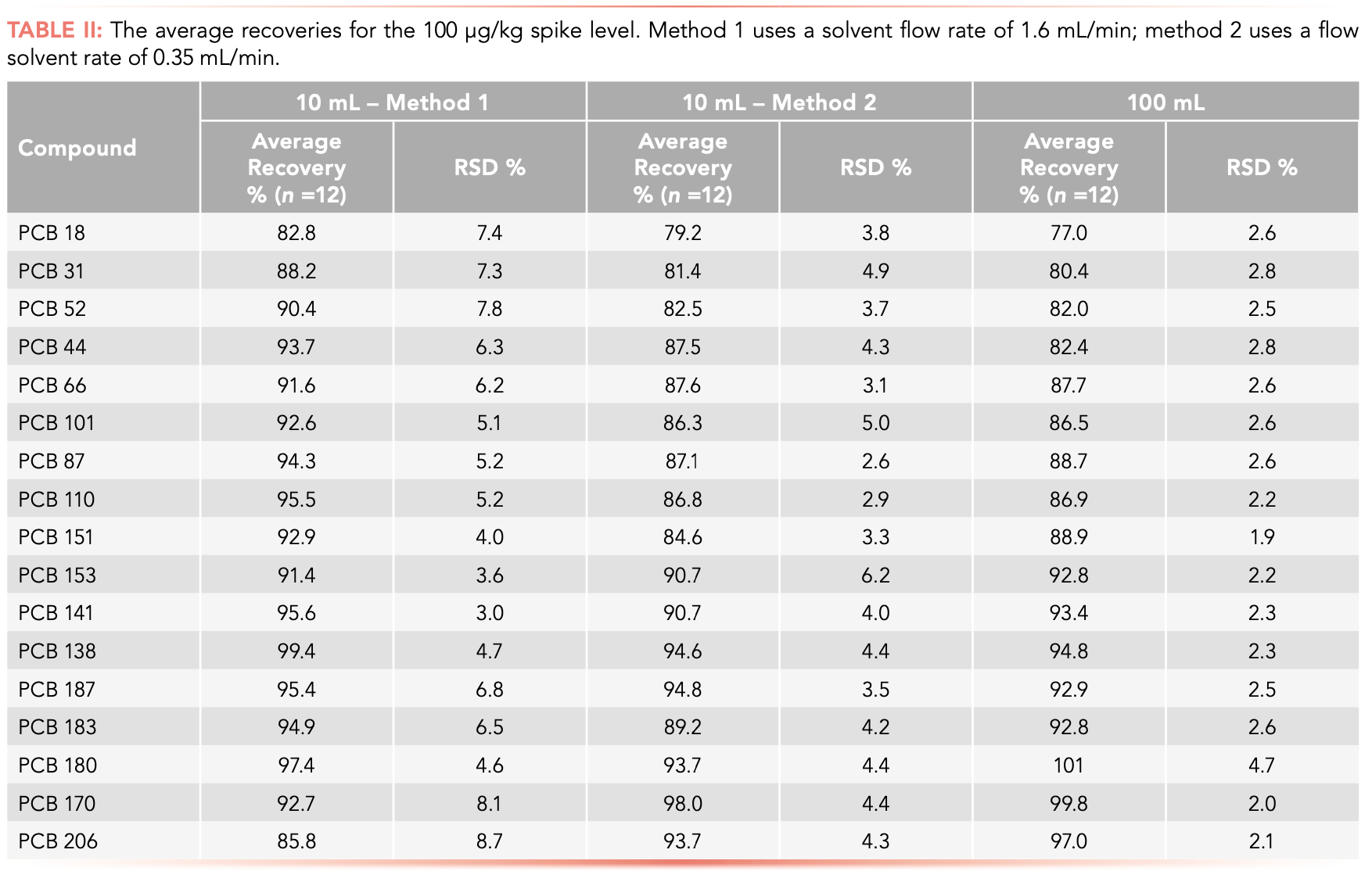
Researchers optimized the workflow further, offering additional efficiencies using the method. Most significantly, they reduced the flow rate from 1.6 mL/min to 0.35 mL/min (Table II, method 2), finding it only minimally affected recovery rates but slashed solvent consumption by half.
PAH Analysis
PAHs are persistent environmental contaminants that arise from the incomplete combustion of organic materials such as coal, oil, petrol, and wood. Hundreds of PAHs are known, but only 16 have been designated as high priority pollutants by the EPA for toxicity, carcinogenicity, and mutagenicity. Also, as PAHs bind to particulate matter, they can be transported long distances away from their origin, further intensifying the need for accurate concentration determination.
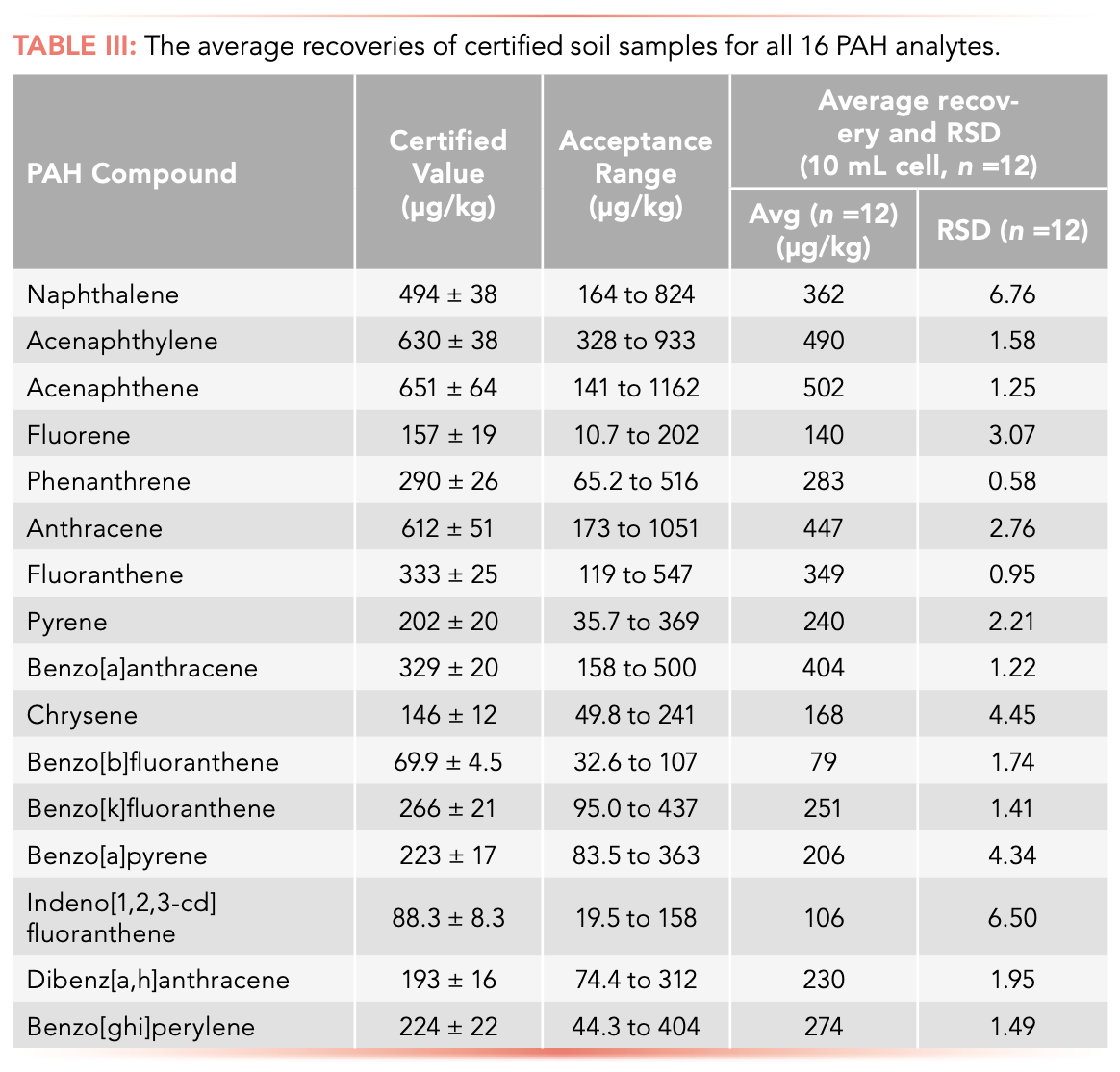
Recently, a study used new ASE methodology to determine the 16 PAHs in soil (4). All the recovery rates of the PAH analytes were between 77.5% and 106.6%, well within EPA requirements. The method was also highly reproducible, with an RSD of below 20% for all compounds investigated.
To further determine the accuracy and precision of the approach, the team performed carryover studies. Less than 0.5% carryover was determined for all analytes, demonstrating the efficacy of rinsing between extractions.
Finally, 12 replicates on certified PT samples all gave RSDs of below 7%, falling within the acceptance range of the accompanying certificate (Table III).
Conclusion
Fast and effective sample preparation is crucial to maintain the highest level of quality in POP analysis, but traditional, manual procedures currently make it time-consuming, resource-intensive and error prone. What’s more, inefficient processes can be laborious and demotivating for analysts, leading to recruitment and retention issues.
Now, sample preparation doesn’t need to be a bottleneck in the analytical workflow. Automating sample preparation and combining extraction and evaporation steps significantly improves the process by making it faster and more cost-effective. In addition, a protocol that runs samples in parallel can improve capabilities further by dramatically increasing sample throughput.
The advanced and automated ASE methods that combine extraction and evaporation enable highly efficient sample preparation for OCBs, PCBs, and PAHs. Three studies demonstrated that the method met the recovery requirements outlined by the EPA for each class of analyte, and all samples had RSDs below 20%, demonstrating the reproducibility of the approach. Additionally, a rinse between extractions can effectively minimize carryover, with less than 0.5% carryover of the analytes detected.
Beyond accuracy and precision in POP analyte determination, automated parallel extraction and evaporation systems also improve sample throughput, while reducing error and hands-on time. Such an automated workflow can improve POP analysis for many laboratories, and result in increased personnel productivity, happiness, and retention.
References
(1) Persistent Organic Pollutants (POPs) and Pesticides. https://www.unep.org/cep/persistent-organic-pollutants-pops-and-pesticides (accessed 2022-12-05).
(2) Ullah, R.; Galbiati, F.; Wang, A.; Qiu, C.; Wang, M.; Liu, Y. Determination of Organochlorine Pesticides (OCPs) in Soils using the EXTREVA ASE Accelerated Solvent Extractor and GC-ECD. https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/an-001054-ic-sample-preparation-extreva-ase-ocps-in-soils-an001054-en.pdf (accessed 2022-12-05).
(3) Wang, M.; Ullah, R.; Galbiati, F.; Meng, X.; Lu, D.; Jiang, P.; Qiu, C. Determination of Polychlorinated Biphenyls (PCBs) in Soils using a New Fully Automated Parallel Extraction and Evaporation System and GC-MS. https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/an-001419-ic-extreva-ase-pcbs-soil-an001419-en.pdf (accessed 2022-12-05).
(4) Qiu, C.; Wang, A.; Ullah, R.; Liu, Y.; Wang, M.; Lu, D.; Galbiati, F. Determination of Polycyclic Aromatic Hydrocarbons in Soils using the EXTREVA ASE Accelerated Solvent Extractor and GC-MS. https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/an-001106-ic-sample-preparation-extreva-ase-pahs-in-soils-an001106-en.pdf (accessed 2022-12-05).
(5) SW-846 Test Method 8000D: Determinative Chromatographic Separations. https://www.epa.gov/hw-sw846/sw-846-test-method-8000d-determinative-chromatographic-separations (accessed 2022-12-05).
Chris Shevlin is Scientific and Educational Affairs Manager, Ion Chromatography and Sample Preparation, at Thermo Fisher Scientific. Rahmat Ullah is Manager, Chemistry for the Ion Chromatography Sample Preparation (ICSP) business unit at Thermo Fisher Scientific. Direct correspondence to: ICSPMarketingManagement@thermofisher.onmicrosoft.com

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.









