Optimizing Column Lifetime of TSK-GEL Size Exclusion Columns
The Application Notebook
Column lifetime is a more and more important issue when developing an analytical method for HPLC. Besides sample treatment, column cleaning and storage, operational parameters of the analytical method will have an influence on column lifetime. This question may not always be addressed early enough in the methods development process.
Introduction
The first factor is the sample itself. Crude, complex samples present more of a challenge for separation and maintenance of the integrity of a column. Any type of sample cleanup prior to analysis will not only provide a better separation but also extend the lifetime of the column.
The second factor is the assay conditions. These conditions are primarily dictated by the need to achieve a robust separation. However, the conditions should be as mild as possible, and within the physical limits of the column. Extremely high or low pH conditions, high temperatures and flow-rates close to the maximum will shorten column lifetime by increasing the possibility of voiding.
Mobile phase additives, such as organics, salts and detergents also play a role in the lifetime of a column by minimizing secondary effects such as ionic and hydrophobic interaction between the sample and the column packing material. Ionic interactions can be suppressed by increasing the ionic strength of the buffer up to a salt concentration of 0.5 M. The addition of acetonitrile, acetone or alcohols up to a concentration of 20% may prevent columns from column fouling by suppressing hydrophobic interaction of the sample. An example is demonstrated below.
The final factor is column maintenance.
The use of a guard column is the first line of defence against sample contaminants, pump seal fragments and any other solids in the mobile phase. Arguments against using a guard column, such as increased run times and additional cost, are outweighted by the overall benefits, as demonstrated.
Results
Mobile Phase
The analysis of a pegylated protein PEG-r-HuMGDF on a TSKgel G3000SWXL column demonstrates the influence of the mobile phase on column lifetime (see Figure 1 ). Pegylated products are more hydrophobic, so they tend to interact with the column matrix. Over time enough of the pegylated product can foul the column, which is indicated by shifts of retention time and an increase of peak tailing. In Figure 1(a) a standard phosphate buffer mobile phase was used. The degradation of column performance was obvious after 150 injections of the PEG-r-HuMGDF. By adding 10% of ethanol to the elution buffer, this problem is overcome. Figure 1(b) shows no differences in performance between the initial and the 150th injection (courtesy of J.J. Ratto et al. Amgen Inc., 1996).
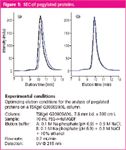
Figure 1.
Column Protection
The influence of a guard column on column lifetime was examined by injecting a very clean protein standard sample on a TSKgel SuperSW3000 size exclusion column. Throughout 4000 injections retention time and efficiency were monitored for the pABA peak. Retention time remained fairly consistent over the course of the 4000 injections but efficiency dropped below 21000 plates after a few hundred injections. The plate count was restored after a new guard column was put into use as depicted in Figure 2. During the course of this experiment five different guard columns were used to achieve the 4000 injections. The used guard columns showed no evidence of voiding when being opened, only a dark residue on the column top, which derived from the pump seals.

Figure 2.
Conclusion
During method development, column lifetime is an issue to which attention must be paid. Assay conditions should follow the vendor's recommendations and should be developed in respect of preventing column fouling by sample contaminants.

Table 1: Factors influencing column lifetime.
The use of a guard filter or guard column, or both should be included as part of the methods development as well.
References
1. J.J. Ratto and S.R. O'Conner, The Peak, a TosoHaas newsletter, Vol. 5, Issue 6, An Ethanol/Sodium chloride/Pogsphate mobile phase for SEC of Pegylated Proteins. Poster presented at HPLC '96, San Francisco, USA.

Tosoh Bioscience GmbH
Zettachring 6, 70567 Stuttgart, Germany
tel. +49 711 132570 fax +49 711 1325789
e-mail: info.sep.eu@tosohbioscience.com
website: www.tosohbioscience.de

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.










