De Novo Formula Generation with "Sub-ppm" Confidence using Compass OpenAccess and the micrOTOF
The Application Notebook
Accurate mass measurements are a key element of chemical characterization. However, the accepted mass accuracy tolerance of 3–5 ppm can still leave significant ambiguity in the proposed chemical formula. Consequently a further input from other analytical techniques such as NMR or MS/MS, along with some judgment based on the synthetic history is often required to arrive at a confident formula assignment.
Accurate mass measurements are a key element of chemical characterization. However, the accepted mass accuracy tolerance of 3–5 ppm can still leave significant ambiguity in the proposed chemical formula. Consequently a further input from other analytical techniques such as NMR or MS/MS, along with some judgment based on the synthetic history is often required to arrive at a confident formula assignment.
Traditionally, visual inspection of isotopic information from magnetic sector MS spectra was useful to eliminate spurious formula suggestions. However, to date ESI-TOF technology has been unable to reproduce accurate isotopic patterns and this valuable chemical information has been lost to the characterization task, making automated formula determination almost impossible.
Novel technology embodied in the micrOTOF allows precise measurement of both accurate mass and true isotopic pattern (TIP) over a wide dynamic range, allowing for the implementation of an open access system. This instrumentation enables chemists with limited LC–MS skills to rapidly confirm and identify pharmaceutical compounds with "sub-ppm" confidence in a fully automated workflow.
OpenAccess Software Design
Compass OpenAccess has been implemented with a client server architecture (Figure 1). All information regarding samples, status of the jobs, data, reports and processing results are stored in an Oracle database. The database, located on a central server, serves multiple instruments and computers. Samples can be submitted easily from any PC in the intranet via a web interface. Also the results can be viewed via a simple viewer in the Internet Explorer.
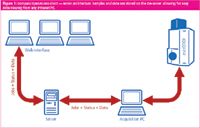
Figure 1.
On the acquisition PC a very simple user interface hides the complexity of the LC–MS system and allows the user to easily queue the submitted samples for automatic acquisition and processing.

Figure 2.
When the sample has been processed, spectra, chromatograms and formula suggestions are sent to the user as a PDF report via e-mail and can also be easily accessed via the web interface (Figure 2 and Figure 3).
Experimental
A set of 113 samples was run with Compass OpenAccess with the goal of unattended formula confirmation. The samples were analysed using an Agilent 1100 liquid chromatography system interfaced with a micrOTOF mass spectrometer under Compass OpenAccess control in a flow injection analysis (FIA) set-up. Lithium formate solution was injected as an external calibration standard.
The data were automatically externally recalibrated using DataAnalysis software. Possible molecular formulae were generated using accurate mass, true isotopic pattern and SigmaFit information with generic atomic search criteria (max. C200 H200 N200 O200 Br3 Cl3 F3 S4, and applying the nitrogen rule). Result filter arguments are SigmaFit < 0.05, ppm tolerance < 5 ppm, maximum 5 formulae suggestion, defined adducts (protonation and sodiated), N2-rule active.

The generated formula assignments were then compared to the recorded formulae.
Results
113 samples were analysed in positive ESI mode using the micrOTOF. Over all average mass error = 1.7 ppm, SD = 4.2 under external calibration.
The sigma interpretation limit was set to 0.05. In mass range from 179 to 821 amu 89% of all results fit into 0.03 sigma limit. All results fit into sigma 0.05.
The relative ppm-error of 3 ppm represents 97% of all results.
ConfirmFormula: For all samples a correct formula result was returned as first suggestion ("top hit"), so the given formula could be automatically confirmed.
FindFormula within limits: For 97% of samples, a correct formula was found as first or second hit suggestion.
Conclusion
De novo formula generation using accurate mass, true isotopic pattern and SigmaFit information allows rapidly confirming and identifying pharmaceutical compounds with "sub-ppm" confidence. This provides a robust base for fully automated formula determination and formula confirmation workflows as implemented in Compass OpenAccess.

Bruker Daltonik GmbH
Fahrenheitstrasse 4, D-28359 Bremen, Germany
tel. +49 421 2205 0 fax +49 421 2205 104
E-mail: sales@bdal.de
Website: www.bdal.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)




