Online LC–GC: The Ultimate “GC Connection”
Multidimensional chromatography combining HPLC and GC, or LC–GC, sounds simple, but several factors complicate this combination, including solvent compatibility, separation time, and sample concentration.
Multidimensional chromatography is gaining in popularity for the analysis of extremely complex samples. In multidimensional chromatography, two or more columns are used, most often in tandem, to effect separations not possible with a single column. In this installment of “GC Connections,” the ultimate connection, multidimensional chromatography with high performance liquid chromatography (HPLC) in the first dimension and gas chromatography (GC) in the second dimension, is discussed. On the surface, this seems simple: Just take a sample of the effluent from the HPLC instrument, and inject it into the GC system. However, several factors complicate this analysis, including solvent compatibility, separation time, and sample concentration. We begin by discussing the basic requirements for multidimensional separations, applied to the liquid chromatography-gas chromatography (LC–GC) combination, followed by discussing some instrumental configurations that have been used for LC–GC, and finish with some representative applications.
Atsu Apedo and Nicholas H. Snow
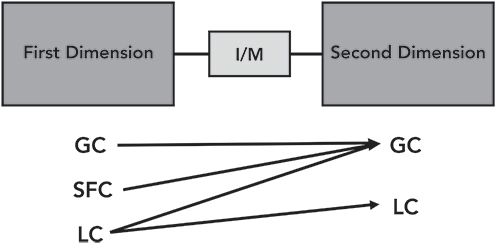
Multidimensional chromatographic techniques involve two (or more) columns, connected, usually in tandem, with a device that allows transfer of one or more aliquots of the effluent from the first column into the second column. This allows transfer of one aliquot or peak at a time, usually termed heart cutting, or continuous transfer, usually termed comprehensive or two-dimensional gas chromatography (GC×GC) (or, if applicable, two-dimensional liquid chromatography [LC×LC]). A simple schematic of a multidimensional chromatography system is shown in Figure 1. Note the labels under each dimension. If the first column is GC or supercritical fluid chromatography (SFC), then the second column will always be GC. If the first column is liquid chromatography (LC), then the second column can be either LC or GC. The interface between the two columns can be as simple as a flow switching valve for heart cutting, or may be a more complex device called a modulator for comprehensive experiments. To achieve an improvement in separation that justifies the more complex instrumentation used in multidimensional separations, the selectivity of the two columns must be sufficiently different.
In multidimensional GC, which has received most of the multidimensional separations attention in the literature, selectivity is achieved by using two columns with very different polarity, most commonly a non-polar column in the first dimension and a polar column in the second dimension. The commonly used term orthogonality is used to describe the degree of selectivity generated by the multidimensional column combination. A new volume on multidimensional GC provides an introduction specific to GC×GC and also covers basic aspects of multidimensional chromatography. The first three chapters address the basics of column configurations and selectivity in detail (1). While GC×GC has received the most attention, LC–GC is possibly the ultimate multidimensional separation technique, due to the selectivity generated by the LC separation being driven by solubility and the GC separation being driven by vapor pressure. These are two very different chemistries, practically guaranteeing the orthogonality desired in multidimensional separations.
Making the LC–GC Connection
How do we transfer a sample from HPLC to GC? One simple way to scout whether a multidimensional LC–GC combination might be useful is to simply collect the HPLC effluent in a fraction collector or a test tube, and then inject a sample of that into the GC instrument. There are several means for performing and automating this process, but, first, some challenges in transferring samples from HPLC to GC must be addressed.
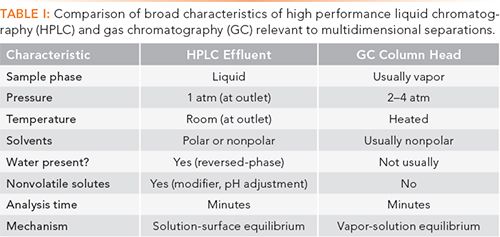
Table I lists several broad characteristics of the HPLC column effluent and GC column head that both assist and complicate their combination in a multidimensional separation. First, in HPLC, the column effluent is in the liquid phase. To be separated in GC, the liquid sample must be vaporized, thus the use of heated inlets in GC. This can, of course, be done using a syringe and injecting the sample into the inlet on the GC instrument, or by using a sample valve to deliver a predetermined volume of the liquid into the inlet. Next, the HPLC column effluent is at room pressure and temperature; in GC, the column head is pressurized and heated. Again, a syringe and the inlet on the GC system easily solve this problem. The biggest challenges in making the LC–GC connection involve the solvents. Most HPLC work is done using reversed-phase columns and polar solvents, including water, and often including ionic modifiers. Solvents such as methanol, acetonitrile, and even water can be injected into gas chromatographs, but they do raise the problems typical with injecting polar solvents onto often nonpolar columns. If polar solvents are used, careful optimization of the injection process into the GC system will be required.
A final problem with LC–GC is the time required for each separation, especially if a comprehensive separation is desired. Generally, for comprehensive separations, the second-dimension separation must be much faster than the first-dimension separation, or the total analysis time becomes prohibitive. In GC×GC, nearly all chromatograms have a first-dimension time in minutes, and a second-dimension time in a few seconds; the second dimension separation is often 500 or more times faster than the first-dimension separation. For LC–GC, in which the HPLC chromatogram and GC chromatogram both require a similar number of minutes, a fraction collector could be used to collect fractions for later injections into the GC system, but this approach requires a time equal to the GC analysis duration times the number of fractions to be analyzed. The HPLC flow can be stopped while the GC separations are performed, but this may result in some band broadening or sample degradation in the HPLC separation, and does not solve the problem of a long analysis time.
LC–GC Interfacing Challenges
First, consider the simplest case: collecting a peak from the HPLC instrument as it is eluted and injecting an aliquot of that peak into the GC system. In this simple, and inexpensive, case, there are several challenges. First, collection of the HPLC sample must be carefully timed using the detector signal to ensure that the peak of interest is collected, requiring an additional data collection channel. Second, the HPLC effluent is diluted. Assume the original total concentration of solutes in the HPLC peak is 100 ppm and the peak volume is 100 µL. This provides a total mass of about 100 ng of solutes in the peak. If 1 µL of this peak is then injected into the GC using splitless injection, it provides a total mass of about 1 ng of analytes injected into the GC instrument. If the peak contains several analytes, they will have masses on the order of picograms, near the lower limits of many detectors. Finally, the solvent itself may be problematic for GC. If normal-phase HPLC is used, the solvents are nonpolar, and generally amenable to splitless injection. If a solvent mixture is used, careful optimization of the injection may be required. If reversed-phase HPLC is used, the polar solvents, including water, are not generally amenable to splitless injection on most capillary columns. Additional techniques such as retention gaps, an additional phase transfer step, or large volume injection techniques, described below, are necessary to prevent poor peak shapes or possible column damage. In both cases, nonvolatile modifiers may not be added to the HPLC mobile phase, or must be removed prior to injection onto the GC column. The problems of timing, analyte concentration, and the nature of the mobile phase were addressed over the years during the development of online LC–GC techniques.
LC–GC Interfacing Solutions
Online LC–GC interfacing has been performed for decades; the first publications appeared in the late 1970s (2,3). As discussed above, the most difficult challenge is the removal of relatively large volumes of often polar solvents. In a 2000 review, Grob discussed early developments with the most common interfaces that are still used today (4). At that time, Grob concluded that the interfacing was best conducted using a large volume injection device for the transfer. For normal phase LC–GC, he suggested use of an on-column inlet combined with a solvent vapor exit device, and for reversed-phase LC–GC, he suggested programmed temperature vaporization (PTV) based large volume injection, using a packing in the liner that is suitable for the more polar solvents. Since 2000, most LC–GC work has used one of these approaches.
Although comprehensive analysis was considered early on, most LC–GC applications involved heart cutting one or a few samples from the HPLC run.
A 2012 review by Purcaro, Moret, and Conte builds on the discussion by Grob, and highlights advances to that point (5). Additional work on the various interfaces is discussed, with the PTV-based approaches gaining in use and mostly overcoming the others. They describe additional developments with reversed-phase LC–GC, with discussion of multiple approaches for eliminating the polar solvents. Both Grob and Purcaro, and their associates, also comment on the need for expertise when considering LC–GC, because both techniques must be optimized to work in concert with the other. For example, Grob discussed that water-containing samples can be transferred from HPLC to GC without special solvent trapping if five conditions are met:
- There are no salts in the mobile phase
- A packed liner is used to trap the solvent and facilitate vaporization
- Sample evaporation and solute/sample separation occur as separate processes
- The vaporizer is heated to facilitate solvent evaporation
- Solute retention does not occur by solvent effects
These requirements place very specific limitations on both the HPLC and GC analyses. For the HPLC analysis, the first requirement states that no modifiers can be used; the mobile phase must include only solvents. Requirements 3–5 state that the GC inlet must be a PTV or a similar device allowing trapping of the full sample, followed by solvent evaporation, and then thermal transfer of the analytes to the column. The final requirement places limits on the type of analyte; if solvent focusing cannot be employed, then the only peak focusing mechanism available is cold trapping (see a recent “GC Connections” article on splitless injection [6]), meaning that the analytes must likely be semivolatile. These restrictions require the user to have significant expertise in HPLC, GC, and the use of a PTV inlet.
In 2013, Mondello and colleagues provided a short review in LCGC North America about LC–GC in food analysis (7). This is an excellent overview of interfacing techniques, focusing on loop-type devices that combine sample loops with an on-column inlet on the GC instrument and syringe type devices that employ a programmed temperature vaporization (PTV) inlet. In both cases, the use of a traditional inlet and traditional columns in the GC have mostly limited the HPLC to normal-phase separations. Both loop-type and syringe-based interfaces can be used for heart cutting and comprehensive applications.
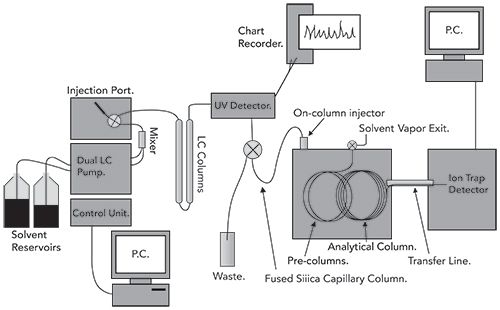
Figure 2 shows a schematic of a loop-type interface from a 1995 application involving analysis of polycyclic aromatic hydrocarbons in urban air particulates, providing some historical context and showing many of the finer points of LC–GC interfacing that might be hidden with today’s more automated systems (8). In a similar application, Hyotylainen and associates used a truly multidimensional approach, combining pressurized liquid extraction with HPLC and GC for analysis of polycyclic aromatic hydrocarbons in sediment (9). In both articles, note the multiple control and data collection points. With the ion trap MS detector, there are really three instruments that must be simultaneously controlled and two (HPLC detector and MS) that require data collection. A six-port valve and an additional sample loop were placed between the HPLC and GC systems to provide a constant volume for injection. To accommodate larger solvent volumes, the GC instrument was configured with a cool on-column inlet with a retention gap and a solvent vapor exit located between the retention gap and the analytical GC column. In 2009, Biederman and Grob described a modification to this technique using a Y-piece, inserted between the HPLC and GC instruments, to reduce memory effects relating to the on-column injection. In 2013, Purcaro and colleagues provided an excellent comparison of the on-column and PTV-based interfaces for analysis of hydrocarbons in food (10,11).
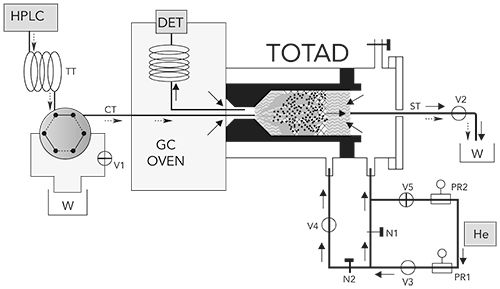
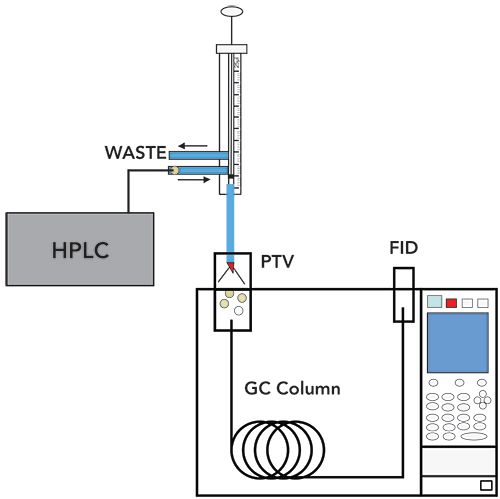
To address the challenges involved with the elimination of polar solvents in reversed phase LC–GC, a through oven transfer adsorption/desorption (TOTAD) interface was developed in 2000, based on a modified PTV inlet (12). Shown in Figure 3, the PTV inlet is oriented horizontally with the HPLC effluent and GC column head both having inlets into a packed bed. HPLC peaks can be captured in the packed bed by reducing the HPLC column flow from 1 mL/min to 0.1 mL/min, with helium flows used to both drive excess solvent to waste and, along with heating, to thermally desorb analytes into the GC column. The expressed need is to thoroughly remove water and polar solvents prior to thermal desorption into the GC column, without also removing analytes from the packing. In Figure 3, the TOTAD device is receiving effluent from the HPLC system. Helium flows in the direction away from the GC column head, allowing the analytes to adsorb on the packing while solvent is vented to waste. Once the analyte fraction is collected on the packing and the solvent is removed, the valves are switched and helium flows into the GC column, carrying the analytes with it. The device was first demonstrated for the analysis of semivolatile pesticides. This device meets the five requirements for transferring polar solvents, outlined by Grob, stated above.
Figure 3: TOTAD Interface. (Adapted with permission from reference 12. Copyright 2000 American Chemical Society).
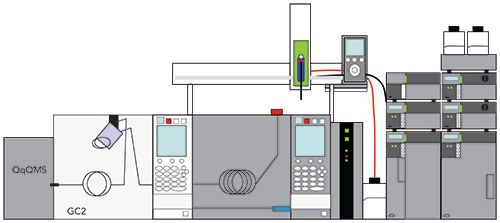
Figure 4 shows possibly the ultimate online LC–GC system more recently described as a proof of concept by Zoccali, Tranchida, and Mondello (13). Excluding the sample preparation, this system shows five dimensions of separations, the HPLC column, first- and second-dimension GC columns, and two-dimensional MS for detection. They used this instrument to analyze coal tar. An 18 min normal- phase HPLC analysis using a silica column was used to generate three fractions: nonaromatic hydrocarbons, aromatic hydrocarbons, and oxygenated compounds. Aliquots of these fractions are then transferred to the GC×GC system, using a commercially available autosampler equipped with a dual side port syringe. The resulting sample for GC×GC is then injected into the GC×GC using a large volume inlet. Interestingly, an instrument constructed in this manner allows nearly all forms of GC and HPLC analysis and their hyphenated combinations to be performed on a single system. With stop-flow in the HPLC, one can easily envision using this system for comprehensive LC×GC.
Figure 5 shows a simplified schematic of an LC–GC system focusing on the interface, which consists of a dual side port syringe and a PTV inlet. When the HPLC system is running and the system is not performing an injection into the GC system, the syringe plunger is all the way in the down position. This forces the HPLC effluent to waste, as usual in an HPLC analysis. To perform the transfer to the GC system, the plunger is raised, and the desired volume of the HPLC effluent is collected in the syringe barrel. The plunger is then lowered to transfer the liquid into the cooled PTV inlet. The inlet is then heated to perform a solvent effect large volume injection to transfer the sample into the GC column.
Example LC–GC Applications
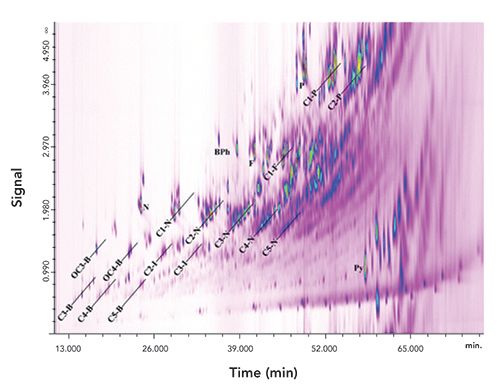
A chromatogram showing the multidimensional GC analysis of a coal tar fraction using the system described in Figure 4 is shown in Figure 6. This demonstrates the high separation power of the LC–GC×GC combination. In this case, the LC system has performed a group-type separation to obtain the aromatic hydrocarbons in a single fraction. GC×GC is then used to separate the individual classes of aromatic compounds. In this chromatogram, the mass spectrometer was operated in full scan mode; if selected ion monitoring or multiple reaction monitoring were used, the detector would allow a further dimension of selectivity. This possibility is addressed by the authors in the original article. As seen from the chromatogram, GC×GC provides a further group-type separation showing separate regions for each class of aromatic hydrocarbon. From the full-scan data, mass spectra can then be obtained for positive identification of any analyte of choice.
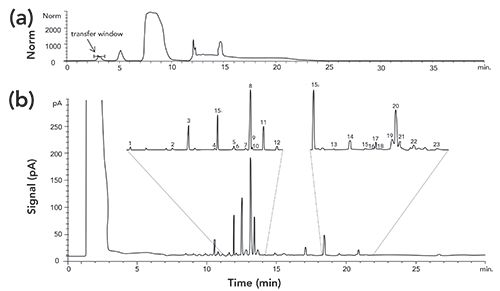
LC–GC has found strong application in the analysis of oils and lipids in foods. The possibility of adulteration of foods by mineral oil or its components has become an important topic in food quality over the past decade (14). This problem also lends itself well to using HPLC for a group-type separation to separate the oil compounds from the food matrix and then to transfer them to the GC for further separation. Figure 7 shows chromatograms for the HPLC and GC analysis of sterols, stanols, and their esters extracted from walnut (15). A common multimode inlet was used to perform PTV solvent vaporization of the HPLC effluent. In the top chromatogram, the HPLC group-type separation is shown, indicating the band that was transferred to the GC instrument. The bottom chromatogram shows the gas chromatographic analysis of the HPLC band with two regions highlighted, showing the separation of 23 analytes.
Figures 6 and 7 demonstrate the most common use of LC–GC: using the LC to generate a preparative group-type separation of particular compound classes (aromatic compounds in Figure 6 and sterols in Figure 7). Both demonstrate one challenge in LC–GC, in that the analysis time in the GC separation is of similar or even longer length than the HPLC separation. This is not a problem if one heart-cut is taken, as in Figure 7,or a few, as in Figure 6. However, this becomes a challenge if a comprehensive separation, as in GC×GC, is desired.
The applications presented here and discussed in the referenced articles have all been traditional LC–GC methods using heart cutting, in which one or a few fractions are collected from the HPLC. Comprehensive LC–GC, in which samples are continuously transferred from the HPLC to the GC, is beginning to see more application. The recent paper by Garcia-Cicourel and colleagues provides an application to mineral oil analysis and a useful introduction to the approach (16).
Conclusions
Online LC–GC interfacing has been performed for over four decades, since the early days of HPLC in the 1970s. When combining HPLC and GC, several challenges related to the mobile phase, sample concentration and analysis time must be considered and overcome. Today, large volume injection techniques are used at the interface between HPLC and GC, with both solvent vapor exit (on-column) and PTV techniques being used and the process automated using a rail-type autosampler. LC–GC can be performed using both normal-phase and reversed-phase HPLC, but most applications currently use normal phase HPLC due to the polar solvents (including water) used in reversed-phase HPLC. LC–GC can be applied in many industries, including food, petroleum, and environmental analyses. LC–GC is a technique of choice in the food industry for the analysis of mineral oil and other hydrocarbon contamination in food. Of all multidimensional chromatography techniques, LC–GC provides the greatest orthogonality; however, it is among the most complex chromatographic techniques, requiring high-level expertise in HPLC, GC, and sample introduction to be successful.
Acknowledgment
This article is dedicated to Atsu Apedo, who passed away suddenly on December 16, 2017, following a brief illness. He was 49 years old.
References
- N.H. Snow, Ed., Basic Multidimensional Gas Chromatography (Elsevier, Amsterdam, The Netherlands, 2020).
- K. Grob, On-line Coupled LC–GC (Huethig, Heidelberg, Germany, 1981).
- L. Mondello, A.C. Lewis and K.D. Bartle (eds), Multidimensional Chromatography (John Wiley and Sons, New York, New York, 2002).
- K. Grob, J. Chromatogr. A 892, 407–420 (2000).
- G. Purcaro, S. Moret, and G. Conte, J. Chromatogr. A 1255, 100–111 (2012).
- N.H. Snow, LCGC North Am. 36(7), 448-454 (2018). http://www.chromatographyonline.com/split-splitless-and-beyond-getting-most-your-inlet-0. (Accessed September, 2019)
- L. Mondello, D. Sciarrone, P. Q. Tranchida, and P. Dugo, LCGC North Am. 30(5), 31–35 (2012). http://www.chromatographyonline.com/advances-multidimensional-LC–GC-food-analysis?id=&pageID=1&sk=&date= (Accessed September, 2019)
- A.C. Lewis, R.E. Robinson, K.D. Bartle, and M.J. Pilling, Environ. Sci. Technol. 29, 1977–1981 (1995).
- T. Hyötyläinen, T. Andersson, K. Hartonen, K. Kuosmanen, and M.L. Riekkola, Anal. Chem. 72(14), 3072–3076 (2000).
- M. Biedermann and K. Grob, J. Chromatogr. A 1216, 8652–8658. (2009)
- G. Purcaro, M. Zoccali, P.Q. Tranchida, L. Barp, S. Moret, L. Conte, P. Dugo, and L. Mondello, Anal. Bioanal. Chem. 405, 1077–1084 (2013).
- M. Perez, J. Alario, A. Vazquez, and J. Villén, Anal. Chem. 72, 846–852 (2000).
- M. Zoccali, P.Q. Tranchida, and L. Mondello, Anal. Chem. 87, 1911–1918. (2015)
- M. Biedermann, C. Munoz, and K. Grob, J. Chromatogr. A 1521, 140–149 (2017).
- R. Esche, L. Muller, and K-H. Engel, J. Agric. Food Chem. 61, 11636–11644 (2013).
- A. R. García-Cicourel, B. van de Velde, J. Verduin, and H-G. Janssen, J. Chromatogr. A (2019) In press, https://doi.org/10.1016/j.chroma.2019.460391.

Atsu Apedo (1968-2017) was a part-time PhD candidate, working on a project related to comprehensive LCxGC at Seton Hall University, where he earned a MS in Chemistry. He was a research scientist at Bristol Myers Squibb for 17 years where he contributed to the discovery of numerous drug candidates and won several awards for his dedication and service. He specialized in method and applications development in HPLC, LC–MS, SFC-MS, GC–MS, LCxGC, GCxGC–TOFMS IC, CE and numerous other techniques. He served as the head of the meals ministry at Mosaic Baptist Church since its inception, formerly as a deacon at Faith Baptist Church and Sunday school teacher at Mosaic Baptist Church, as leader of the church service held monthly at Saint Lawrence Rehabilitation in Lawrenceville, NJ, as leader of the Friday Fellowship, as the former leader of the Nanjing Fellowship in China, and other ministry positions.

Nicholas H. Snow is the Founding Endowed Professor in the Department of Chemistry and Biochemistry at Seton Hall University, and an Adjunct Professor of Medical Science. During his 30 years as a chromatographer, he has published more than 70 refereed articles and book chapters and has given more than 200 presentations and short courses. He is interested in the fundamentals and applications of separation science, especially gas chromatography, sampling, and sample preparation for chemical analysis. His research group is very active, with ongoing projects using GC, GC–MS, two-dimensional GC, and extraction methods including headspace, liquid–liquid extraction, and solid-phase microextraction.
New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)