Olive Oil Quality and Authenticity: Recent Improvements in Sample Preparation and GC–FID Analysis
The Column
The growing popularity of extra virgin olive oil (EVOO), thanks in part to its presumed health benefits, has resulted in an increased incidence of product adulteration to achieve higher financial gains. This adulteration, through dilution with less expensive oils, has created demand for product authenticity testing. Olive oil testing based on traditional methods is slow, labour-intensive, and requires large amounts of organic solvents. This article reviews the challenges in the accurate analysis of olive oil and discusses new methods that can improve this testing.
Photo Credit: Ridkous Mykhailo/Shutterstock.com

David C. Kennedy and Allen Misa, Phenomenex, Torrance, California, USA
The growing popularity of extra virgin olive oil (EVOO), thanks in part to its presumed health benefits, has resulted in an increased incidence of product adulteration to achieve higher financial gains. This adulteration, through dilution with less expensive oils, has created demand for product authenticity testing. Olive oil testing based on traditional methods is slow, labour-intensive, and requires large amounts of organic solvents. This article reviews the challenges in the accurate analysis of olive oil and discusses new methods that can improve this testing.
The consumption of extra virgin olive oil (EVOO) has been broadly linked with dietary protection from heart disease and cancer (1,2). These health benefits along with strong culinary appeal have led to a worldwide surge in demand for EVOO. However, owing to the growing demand for EVOO and to its high intrinsic value, there is a very strong economic temptation all along the supply chain to dilute pure EVOO with less expensive edible oils and pass off the adulterated product as genuine. Consequently, the economically motivated adulteration (EMA) of EVOO has become a major financial and regulatory issue.
As well as outright adulteration, olive oil quality varies extensively depending simply upon the source of the olives, the method of processing, the age of the product, and other common factors. These variables result in a range of composition characteristics that cannot be reliably and repeatedly measured by common organoleptic tests such as taste and odour. To properly characterize the quality and authenticity of olive oil, rigorous analytical testing is required. However, as we shall see, this is not a simple matter.
The Analytical Challenge
EVOO and other edible oils are extremely complex mixtures of fatty acid esters that are closely related, both functionally and structurally. The most successful approach to characterize these oil blends has been the saponification, methylation, and separation of the large number of component fatty acid methyl esters (FAMEs) by high-resolution gas chromatography with a flame ionization detector (GC–FID). FAME analysis has become a widely used analytical tool for distinguishing the quality of edible oils such as EVOO. However, while this approach is useful for characterizing the quality profile of individual olive oils, traditional FAME analysis lacks the resolution to unequivocally identify adulterated EVOO, particularly at low levels of contamination.
Fortunately, another analytical approach is available for assuring EVOO authenticity for both the determination of sterol content and the profile. EVOO is produced at an ambient temperature by a mechanical extraction process that results in a sterol profile that is quite distinct from that of refined oils (even refined olive oils), which are subjected to heat and chemical processing.
The Need for Improvement
A combination of FAME analysis and sterol profiling are generally adequate to establish EVOO purity and authenticity. However, both techniques are based upon separation technology that is decades old. Consequently, olive oil testing based upon these traditional methods is slow, labourâintensive, and requires large amounts of organic solvents. These tests are not optimal to provide the rapid, highâthroughput screening that is needed to effectively meet the growing demand for characterizing olive oil quality and authenticity.
Recent advances in gas chromatography column design and parallel improvements in sample preparation techniques have combined to provide the basis for dramatically improving the performance of both FAME analysis and sterol profiling. As these techniques are further developed and validated, the rapid and economical certification of EVOO purity and authenticity will potentially become a reality.
New Analytical Methodology: FAME Analysis
As noted earlier, the measurement of the fatty acid composition of olive oil using FAME analysis is commonly performed to characterize quality and authenticity. FAME analysis typically uses a highly polar GC column to separate the resulting mixture of fatty acid methyl esters. Generally, a very long column length (100 m) is required to achieve adequate resolution of all the cis and trans isomers on the wide variety of aliphatic carbon chains. These long column lengths result in run times of 60 min or longer.
This article demonstrates the use of a GC column with targeted selectivity that allows for excellent FAME separation using much shorter column lengths, often 30 m or less, to achieve complete resolution. This permits shorter analytical run times, delivering accurate results more rapidly and increased sample throughput.
Materials and Methods
Regular and extra virgin olive oil samples were procured from a local supermarket in Torrance, California, USA. IOC official method COI/T.20/Doc. No 25 was followed for sample preparation, saponification, esterification, and GC instrumentation parameters.
Solid-Phase Extraction (SPE) Method: Cartridge: Strata Si-1, 1 g/ 6 mL (part no.: 8B-S012-JCH) (on vacuum or positive pressure manifold) (Phenomenex); wash: 6 mL hexane; load: oil solution (0.12 g of oil in 0.5 mL of hexane); elute: 10 mL of hexane–diethyl ether (87:13); dry down: evaporate eluate under nitrogen, steady stream; dissolve: purified oil residue in 1 mL hexane; add: 0.1 mL 2N potassium hydroxide in methanol; shake: cap tube and shake vigorously for 15 s, leave to separate until upper layer becomes clear; extract: upper layer for analysis (the heptane solution is suitable for injection into the GC).
GC Conditions: Column: 30 m × 0.25 mm, 0.20âµm Zebron ZB-FAME (7HG-G033-10 Phenomenex); injection: split 50:1 @ 240 °C, 1 µL; carrier gas: helium @ 1.2 mL/min (constant flow); oven programme: 100 °C for 2 min to 140 °C @ 10 °C/min to 190 °C @ 3 °C/min to 260 °C @ 30 °C/min for 2 min; detector: FID @ 260 °C; sample: analytes are diluted 5:1 in heptane. Note that chromatograms for EVOO and olive oil are the same for FAMES profile.
Results
As shown in Figure 1, good separation was achieved for nine FAMEs isomers in olive oil (all nine isomers are present in both EVOO and regular olive oil). A short 30-m length column achieved a 20-min run time while maintaining separation of the cis and trans isomers. This offers an approximately 50% time saving over the 50–60-m columns traditionally required for this analysis.
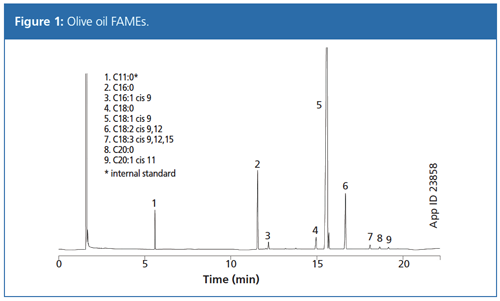
New Analytical Methodology: Sterol Profiling
The International Olive Council (IOC) has established a number of methods for detecting adulterants and determining olive oil quality to guarantee authenticity and safety. In addition to FAME analysis, the determination of sterols in olive oil is another valuable method. A sterol profile provides critical information to distinguish virgin olive oil from refined oils. It also allows the detection of several seed oil adulterants and can even distinguish geographical origin (3). The official IOC method uses saponification of an oil sample followed by liquid–liquid extraction (LLE). Additional sample cleanup is conducted with thin-layer chromatography (TLC). The sterol and triterpene diol fractions are scraped off the TLC plate, then extracted, derivatized, and analyzed by GC–FID.
While the IOC procedure produces reliable data, many aspects are less than ideal from a productivity standpoint. The LLE portion of the procedure is very labourâintensive and consumes large amounts of solvent. Also, it is prone to emulsions, and includes repeated, timeâconsuming washing steps. The TLC portion of the procedure is also timeâconsuming and suffers from low throughput and modest recoveries (4).
Recently, however, a significant improvement to the official sterol method was developed at the University of California, Davis Olive Center (5). In the revised procedure, the LLE step was replaced by supported liquid extraction (SLE) and the TLC step was replaced by solid-phase extraction (SPE). The GC–FID step of the procedure remains unchanged from the IOC procedure. The improved method eliminates many of the problems mentioned above and allows multiple samples to be prepared in parallel. However, the modifications maintain the chemistry of the IOC procedure, allowing direct comparison of analytical results between the new procedure and old. The work described below shows the utility of the improved method for determining sterols erythrodiol and uvaol in olive oil.
Materials and Methods
Extra virgin olive oil (California Olive Oil Council certified) and canola oil were purchased from a local market in Torrance, California. The surrogate for adulterated olive oil used was a 50:50 mixture (v/v) of extra virgin olive oil and canola oil.
Experimental Conditions
Sample Preparation:
Saponification: A 200 mg olive oil sample was added to the test tube containing the internal standard. A 1.5 mL measure of 2 M potassium hydroxide in 95% ethanol was then added to the mixture. The tube was then capped and heated in an 80 °C oven for 25 min. The sample was mixed gently to ensure homogeneity (sample should appear as clear solution) and was heated for an additional 25 min. After heating, 13.5 mL of deionized water was added and mixed.
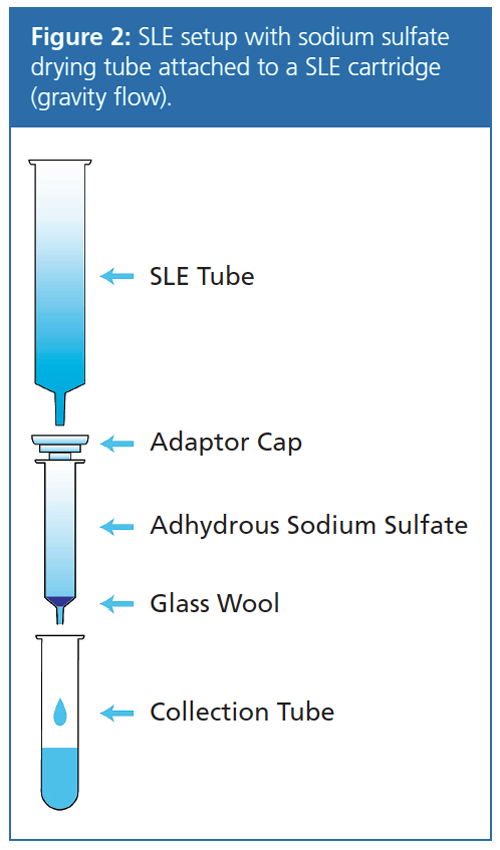
Supported Liquid Extraction (SLE) Protocol: Cartridge: Strata-DE SLE cartridge, 20âmL loading capacity (Phenomenex); load: diluted sample onto SLE cartridge (from saponification step 5) plus 2× 1 mL DI water rinse (gravity flow); wait: 15 min; extract: 3× 15 mL diethyl ether (gravity flow); evaporate: dried under nitrogen flow at 40 °C (greenish-yellow, oily residue); reconstitute: 5 mL of hexane. The SLE tube was rinsed additionally with 2× 1 mL of hexane (rinse was added to 5 mL reconstituted sample prior to loading on SPE tube).
Solid-Phase Extraction (SPE) Protocol and Derivatization: Cartridge: Strata Siâ1, Si-1 (1 g/6 mL) tube (8B-S012-JCH) (Phenomenex); wash: 6 mL hexane; condition: 1. 2 × 6 mL hexane, 2. 1 mL 0.2 M potassium hydroxide in 95% ethanol; equilibrate: 5 mL of hexane immediately after potassium hydroxide elution; load: reconstituted SLE extract plus hexane rinse (7 mL total volume); wash: 85 mL hexane–diethyl ether (98:2) under 3” Hg vacuum, flow rate of 2 mL/min*; elute: 10 mL hexane–ether (60:40); evaporate: dry under nitrogen flow at 50 °C (after dry down, add 3–4 drops mL of acetone and then re-evaporate under nitrogen to remove any occluded water); dry: 10 min in 1000 °C oven; derivatization: 250 µL pyridine–BSTFA (3:1) at 80 °C for 30 min. *A 60-mL empty reservoir tube was attached using an adapter cap to the 6 mL SPE tube to handle the large volume.
GC Conditions: Cartridge: 30 m × 0.25 mm, 0.25-µm Zebron ZBâ5PLUS (7HG-G032â11) (Phenomenex); injection: split 5:1 @ 280 °C, 1 µL; carrier gas: helium @ 0.9 mL/min (constant flow); oven programme: 260 °C for 70 min; detector: FID @ 300 °C; samples: analytes were derivatized with BSTFA in pyridine (1:3).
Results and Discussion
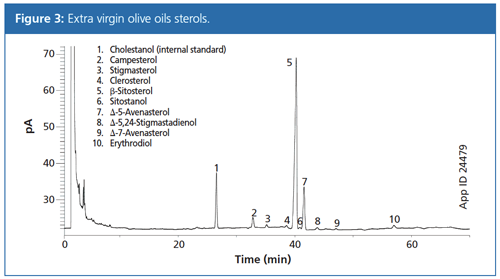
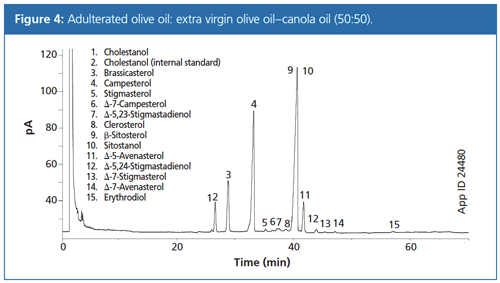
Figure 3 presents the chromatogram obtained from a sample of extra virgin olive oil using the method described. All peaks were well resolved and were identified through a combination of GC–MS chromatograms of analytical standards and by comparing the relative retention times (analyte–β-sitosterol) to literature values. As in the IOC method, it was necessary to use a long run time and isothermal conditions were found necessary to obtain good analyte separation. The extra virgin olive oil sample chromatogram was qualitatively distinguished from that of the adulterated sample chromatogram (Figure 4) simply by observing the presence of extra peaks resulting from the presence of canola oil. In addition, the analyte peaks are quantifiable and allow for classification of oil quality according to the IOC standards for purity. These measured IOC olive oil criteria parameters are summarized in Table 1 along with the relevant sample results.
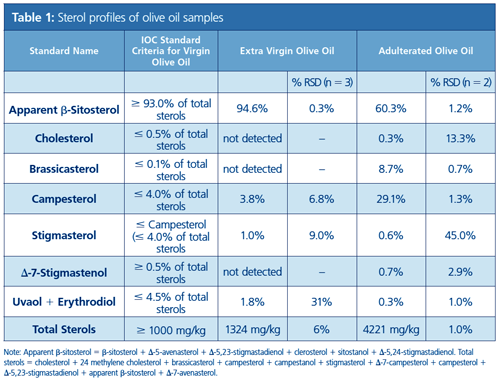
Method accuracy was assessed by analyzing EVOO samples (n = 2) fortified with six reference standards. Recoveries for the analytes brassicasterol (82%), campesterol (75%), stigmasterol (77%), β-sitosterol (100%), erythrodiol (134%), and uvaol (118%) were obtained. The results compared very favourably with the reported recoveries using the IOC method. The method results were reproducible in terms of chromatogram appearance and quantification.
The use of SLE in a large-volume format followed by SPE was found to be an effective and relatively rapid method for determining sterol, erythrodiol, and uvaol concentrations in olive oil. Method accuracy was demonstrated by recoveries of six selected analytes ranging from 75% to 134%, which are comparable to or better than those reported using the IOC method. Importantly, the SLE and SPE method allowed clear, quantitative distinction between extra virgin olive oil and adulterated olive oil. The EVOO samples passed all seven IOC sterol criteria and the adulterated sample failed four. Additionally, the improved method eliminates the need for large glassware, which must be cleaned between analyses. The revised method processed up to 16 samples in parallel over two days, including the GC–FID analysis. Although the method has been significantly improved, there are additional opportunities for further improvements to decrease analysis time and increase productivity
Conclusion
This article has demonstrated the value of modernizing long-established analytical methods to take advantage of the improved performance of new chromatographic techniques. Unfortunately, reference methods are slow to change, owing to the requirement to fully validate multi-laboratory performance equivalency. However, the widespread concerns about EVOO adulteration have created a political and regulatory climate in the United States that may overcome this inertia.
References
- European Food Safety Authority, EFSA J.9(4), 2033 (2011).
- L. Schwingshackl and G. Hoffmann, Lipids Health Dis. 13(1), 154 (2014).
- N. Tena, S.C. Wang, R. Aparicio-Ruiz, D.L. García-González, and R. Aparicio, J. Agric. Food Chem.63, 4509–4526 (2015).
- S. Azadmard-Damirchi and P.C. Dutta, J. Chromatogr. A 1008, 183–187 (2006).
- B. Mathison and D. Holstege, J. Agric. Food Chem. 61, 4506–4513 (2013).
David C. Kennedy is Business Development Manager for Phenomenex. He is a graduate of Iowa State University with a B.Sc. in chemistry and a Ph.D. in analytical chemistry. His professional career has spanned over 45 years with a focus on food safety and environmental monitoring. He has had sequential assignments in industrial R&D, contract testing laboratories, and in the manufacture of analytical instruments and consumables. He is a board member at large with the Pacific Southwest Section of AOAC International.
Allen Misa holds an M.Sc. from California State University, Long Beach, along with a B.Sc. in microbiology from California State Polytechnic University, Pomona. He is the Food and Environmental Marketing Manager for Phenomenex.
E-mail: AllenM@phenomenex.com Website: www.phenomenex.com

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.









