Novel Sorbents for Solid–Liquid Extraction
LCGC North America
Some solid–liquid extraction sorbents that have had significant development recently include MIPs, dSPE for QuEChERS, nanomaterials, and mixed-mode and silicone monolith sorbents.
New sorbent materials for solid-phase extraction (SPE), dispersive solid-phase extraction (dSPE), solid-phase microextraction (SPME), and other solid–liquid extraction methods continue to be developed both in academia and in the commercial sector. Here, we cover some areas of significant development, including molecularly imprinted polymers (MIPs), dSPE for QuEChERS, nanomaterials, and mixed-mode and silicone monolith sorbents.
Solid–liquid extraction is probably the most popular method for sample preparation in the chromatography laboratory. Solid–liquid extraction techniques such as solid-phase extraction (SPE), solid-phase microextraction (SPME), stir-bar sorbent extraction (SBSE), and dispersive solid-phase extraction (dSPE) continue to see increased application in diverse markets such as environmental, food and food safety, forensics, and the life sciences. As sample sizes are continually decreased, more-selective sorbents are required to eliminate matrix compounds that may cause interference in the analytical measurement. With the increased use of liquid chromatography–tandem mass spectrometry (LC–MS-MS) and gas chromatography–tandem mass spectrometry (GC–MS-MS), the potential for ion suppression and ion enhancement, which affects signal intensity, continues to draw attention to sample preparation technologists. In this installment, I cover some newer sorbent materials that have the potential to help in solving specific laboratory problems. In next month's installment, I will look at entirely new sample preparation technologies that may break away and become the next technique to help in eliminating the laboratory sample preparation bottleneck.
Molecularly Imprinted Polymers
Molecularly imprinted polymers (MIPs) are among the most selective phases used in SPE. The technique is sometimes referred to as molecularly imprinted solid-phase extraction (MIP-SPE). Molecular imprinting is a technique that has been used in areas where selective recognition is required for complex separations or sample cleanup. An introductory article (1) outlines the basics of MIP technology, and review articles (2–5) and several books (6–8) provide detailed information on the use and potential of MIPs in SPE.
A MIP is a highly stable polymer that possesses recognition sites that are adapted to the three-dimensional shape and functionalities of an analyte of interest. The most common approach through the use of noncovalent imprinting involves a print molecule (template) that is chemically coupled with one of the building blocks of a polymer. After polymerization, the resulting bond must be cleaved to obtain a free selective binding site (receptor). The synthesis process is shown schematically in Figure 1. The selective interactions between the template and the monomers are based on hydrogen bonding and ionic or hydrophobic interactions. The most often used monomers are based on methacrylic acid or methacrylates. The basic idea of a MIP is the "lock and key" concept in which a selective receptor or cavity on the surface of a polymer perfectly fits the template analyte that was used to prepare the MIP. The receptor site is complementary to the template in terms of its size, shape, and functionality. The concept is similar to immunoaffinity SPE phases, but obtaining and linking a suitable antibody for these immunoaffinity sorbents can be very time consuming and expensive.
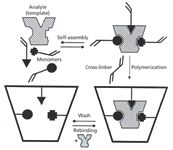
Figure 1: Synthesis of a molecularly imprinted polymer stationary phase.
The removal of the template from the polymeric MIP is important not only to make the interaction sites available for increased sample capacity, but also to ensure that the analyte to be isolated can be measured quantitatively. The lack of removal of the template molecules, even with exhaustive extraction, has been one of the main problems with the acceptance of MIPs. The template molecules frequently bleed, sometimes give baseline drifts, and interfere with the quantitation of the desired analyte, especially at low levels. One approach to overcome this limitation is to use a template that is similar to the analyte of interest. An example would be to use a brominated analog template rather than a chlorinated molecule of interest. If the analog can be separated from the analyte of interest, then the MIP will function as desired.
With aqueous mobile phases, MIPs can display reversed-phase and ion-exchange interaction because selective polar interactions are impaired. The MIP phases show the greatest selectivity when the experimental conditions are chosen that generate the selective interactions that are usually obtained in organic solvents used for the MIP synthesis. This approach allows the MIP to be used for trapping analytes from aqueous solution by hydrophobic or ionic interactions, then washed with a solvent that breaks selective binding of matrix components, and finally with an organic solvent that disrupts the strong bonds between the analyte and the MIP polymer matrix.
Because the SPE packing material is a polymer, depending on the degree of crosslinking there may be some swelling or shrinkage with a change in solvent. Such a physical change can modify the size of the receptor and change the selectivity of the MIP for the target analyte. In this regard, perhaps the synthesis of molecularly imprinted organic–inorganic hybrid polymers (9) may generate a more rigid substructure that does not swell and shrink.
A disadvantage of the MIP approach to SPE is the fact that each sorbent must be custom made. One determines the specificity of the MIP by choosing the appropriate template molecule. The MIP can be synthesized in the laboratory using published procedures, or the template molecule can be sent to a specialty laboratory that will make a custom MIP. Because of the relatively long process involved in making a MIP for SPE, one can justify it only if the application will frequently be required or if there is no other way to perform sample cleanup.
Off-the-shelf MIPs have been introduced. These standard MIP phases have been designed for specific analytes that are popularly encountered in complex matrices. Among those currently available from Supelco and Biotage are sorbents optimized for
- clenbuterol in biological fluids
- beta agonists: multiresidue extractions in urine and tissue samples
- NNAL (4-methylnitrosamino-1-[3-pyridyl]-1-butanol): tobacco-specific nitrosamine in biological matrices
- riboflavin (vitamin B2) in aqueous samples
- triazines: multiresidue extraction in water, soil, and food products
- chloramphenicol: antibiotic in biological matrices
- beta blockers: multiresidue extractions in water and biological samples.
Novel MIP Synthesis
MIPs are generally prepared by traditional bulk polymerization and must be ground and sieved to have the desired particles be in an appropriate size for use. This tedious and time-consuming process often produces particles that are irregularly shaped and thus do not pack as well as spherical particles might. Monolithic polymers consist of a continuous bed with no interstitial volume but only internal porosity. Monolithic beds result in fairly homogenous flow characteristics with a low pressure drop. By combining the MIP technology with monolithic technology, SPE and high performance liquid chromatography (HPLC) columns can be prepared that provide the advantages of both technologies (10–12). Yan, Qiao, and Ho Row (12) synthesized a MIP-monolithic column and used it for the selective on-line SPE extraction of enrofloxacin and ciprofloxacin from urine. Enrofloxacin is a fluoroquinolone with a broad antibacterial spectrum and high bacterial activity against major pathogenic bacteria. Its primary metabolite is found in diseased animals. Because this synthesis of the MIP-monolith was carried out in an aqueous environment (5:1 methanol–water as a porogenic solvent), a high specificity for enrofloxacin and ciprofloxacin in aqueous systems was obtained. Common to many MIP synthesis, to prevent template leakage, a structural analog of each target molecule was used to prepare the imprinted polymers. The downstream HPLC separation was sufficient to separate the small amount of template from the enrofloxacin and ciprofloxacin peaks when the sample was eluted onto the HPLC column. One beauty of monolithic columns is that direct injection of liquid samples is frequently possible. In this case, the authors were able to inject 0.5 mL of a spiked urine sample directly onto the on-line SPE column via a column switching setup. There were no apparent deleterious effects on the MIP, but a washing step was used to ensure that there was no interference from any matrix compounds in the urine. The method showed good linearity, acceptable accuracy and precision, and recoveries in excess of 88% at three spike levels.
To make a spherical MIP specific for triazine herbicides, Matsui and colleagues (13) used suspension polymerization. The synthesis was performed in a two-phase system consisting of chloroform–atrazine (the template)–ethylene glycol dimethacrylate and azobis(dimethylvarelonitrile), which was poured into water containing poly(vinyl alcohol). After a considerable time of heating and stirring, the resultant particles were filtered and thoroughly washed with methanol–acetic acid to remove the template. A nonimprinted polymer (NIP) was also prepared to compare the MIP to a similar polymer prepared without a template present. Although several triazine herbicides were used to show retention characteristics, simazine was chosen as the analyte for actual samples with various spiked impurities. However, when the simazine and impurity mix was applied to the MIP and washed with dichloromethane only the simazine remained behind, indicative of the strong binding based on molecular recognition. Methanol was then used to elute the simazine. Recovery was determined to be 91%. When a NIP was used as the sorbent, the capacity factor for simazine was only 10% versus the MIP indicating the greatly increased MIP specificity for the herbicide.
Commercial stir bars for SBSE are coated with polydimethylsiloxane, which is apolar in nature. A novel twist of combining a MIP with a stir bar was developed by Chen and colleagues (14). A stir bar with a selective coated phase represents a fairly simple approach to selectively enrich trace analytes in a liquid sample. The stir bar just has to be dropped into a solution and the stirrer turned on for a finite period of time, usually in the range of 30–45 min. In this case, the workers prepared a bensulfuron-methyl (BSM) imprinted polymer monolith on a glass stir bar as well as a nonimprinted polymer monolith. Besides BSM, both stir bars were used to extract several sulfonylurea herbicides from water. After extraction, the adsorbed analytes were released by immersing the stir bars in an acetonitrile–trifluoroacetic acid solution. After the stir bar was removed, the solvent was evaporated to dryness and reconstituted in acetonitrile and injected into an HPLC system equipped with a UV detector. For BSM, the selectivity factor for the MIP-coated bar was 260% of the nonimprinted bar. For several other herbicides similar in structure to BSM, the MIP stir bar also showed a selectivity factor of around 30% higher than the nonimprinted stir bar, indicating that there is also some enhancement for similar compounds. The MIP stir bars were used at least 150 times without any ill effects.
Some similar studies were performed with a molecularly imprinted nylon 6 (polycaprolactam) stir bar for the enantioseparation of amino acids. Zhu and Zhu (15) synthesized a stir bar with a high affinity for L-glutamine compared to its isomer and analogues. A reference NIP stir bar showed no selectivity toward the enantiomers. Scientists in Spain (16) have developed a procedure using a MIP stir bar that will extract the fungicide thiabendazole from citrus fruits. They used chemical coating by first derivatizing the stir bar with a silylating reagent and immersing it in a mixture of methacrylic acid and crosslinkers containing thiabendazole as the template molecule. The template was removed by washing with a solution of acetic acid-methanol. After the MIP stir bar was immersed in homogenized extracts of fruit peels or pulps treated with thiabendazole for 45 min, the stir bars were removed, rinsed, and the analyte was released with methanolic acetic acid, the extracts were analyzed for the fungicide by HPLC with fluorescence detection.
As mentioned earlier, a common problem with MIPs is the unextractable template remaining behind in the polymer matrix. Despite exhaustive extraction of the MIP by Soxhlet, ultrasonic, or other normal methods, traces of template still remain. These traces often leach (or bleed) from the template, thereby fouling up quantitation. Working around this involves coming up with a template similar to, but not the same as the target compounds. This bleeding template is intended to be separated by the HPLC or GC separation step after the analyte enrichment. Batlokwa and colleagues from the Chemistry Department at Rhodes University in South Africa (17) came up with a novel solution to the template removal problem that uses pressurized hot water extraction. Surprisingly, as water is heated above its boiling point there is a steady decrease in its permittivity, viscosity, and surface tension as well as an increase in its diffusivity characteristics. With enough pressure to maintain water in the liquid phase at elevated temperatures, its dielectric constant decreases so that it has the "solvent power" of methanol or ethanol, depending on the temperature and pressure. Moreover, water is a safe, nontoxic, easily obtained, and easily disposed of solvent and when used for extraction it decreases the use of organic solvent. Using three different, distinctly colored MIPs, template removal was investigated using hot water extraction and UV absorbance. Washing conditions were optimized for the three MIPs and it was observed that extraction efficiency of template removal was more than 99.6% (compared to <94.5% for Soxhlet and ultrasonics) with no subsequent or minimal template bleed (<0.01%). The extraction of template was not only more complete, but also faster than Soxhlet and ultrasonics (70 min compared to 18 h). So, this safe and low-cost technique may go a long way to reduce the bleeding template problem for MIPs.
Removal of Pigments in Dispersive SPE for QuEChERS
By now, many chemists are familiar with the principles of QuEChERS (quick, easy, cheap, effective, rugged, and safe). The use of QuEChERS has become a standard sample preparation for pesticide analysis in fruits and vegetables and beyond (18). Earlier, we discussed the basics of the technique (19) as well as interviewed the inventors on the latest developments (20). Basically, QuEChERS consists of two steps: salting out extraction and dispersive solid-phase extraction. Both extractions are based on the use of acetonitrile as an extracting solvent for analytes of interest, with the intention of leaving the matrix compounds behind. In the dSPE step, a sorbent is chosen that will retain matrix compounds and leave the solutes of interest in solution. For example, for samples with high fat content, primary–secondary amine mixed with C18 sorbent is recommended; for samples with moderate, and high levels of chlorophyll and carotenoids (for example, spinach, carrots), primary–secondary amine is mixed with graphitized carbon black (GCB) to reduce these colored compounds. Although the addition of GCB helps with the partial removal of chlorophyll and other pigments, there is an accompanying partial loss of certain structurally planar pesticides such as carbendazim and cyprodinil. It is believed that the increased adsorption of planar pesticides in GCB is caused by the platelet structure of the carbon where the pesticides can be tenaciously held between the layers. The planar pesticide recovery can be increased by the addition of toluene in the extraction solution (21); however, toluene may have an impact on the successful extraction of other compounds in the solution. Newer sorbents that will remove chlorophyll without adsorbing the planar pesticides have been under investigation and some of the newer approaches are reported here briefly.
Carbon on Silica
Although GCB is an excellent sorbent for removing pigments and other hydrophobic compounds during the dSPE step, it is quite difficult to work with. It is light and fluffy, difficult to pack, sticks to the walls of the extraction vessel, may carry over to the injection into the HPLC or GC column, and makes everything it touches turn black. United Science has developed a material called CarbonX for QuEChERS that consists of carbon deposited on high-surface-area porous substrates (for example, silica and alumina). The material is designed to provide higher recoveries of the planar pesticides without the handling problems of GCB and without the use of toluene. The material is denser and structurally stronger than GCB and provides high recovery for pesticides in pigmented fruits and vegetables, such as broccoli, spinach, red pepper, and strawberries. Figure 2 shows that CarbonX yields much higher recoveries of planar pesticides than does GCB without the use of toluene to desorb them from the carbon phase (22). Besides use as a bulk material in dSPE, the media can also be packed into a column (cartridge) and used for regular SPE experiments.
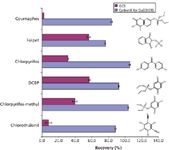
Figure 2: Comparison of planar pesticide recoveries after cleanup with CarbonX (United Science) for QuEChERS and graphitized carbon black (GCB). Reproduced with permission from reference 22.
MIP for Chlorophyll
As was discussed earlier, MIPs are generally used for removing selected analytes from complex matrices. MIPs also might be used for removing matrix compounds instead. A novel MIP was prepared by Batlokwa and colleagues (23) that was selective for chlorophyll, a common pigment in green plant extracts. Chlorophyll must be removed or substantially reduced before injection into a chromatographic column. To prepare the chlorophyll MIP, chlorophyll a, methacrylic acid, ethylene glycol dimethacrylate, and azobisisobutyronitrile in the mole ratio 1:5:25:0.4 were dissolved in dichloromethane and refluxed in an oil bath at 75 °C for 9 h. The resultant rigid polymer was ground to a powder and the template was removed by pressurized hot water extraction as described earlier. A NIP was also prepared. The materials were compared to GCB in QuEChERS experiments. For an equivalent reduction in chlorophyll in a standard buffered solution (2% w/v), 850 mg of the chlorophyll MIP had to be used compared to 300 mg of GCB. For planar pesticides, the MIP did not show any selective binding and the percentage of chlorophyll removed was more than 99.75%. The percentages bound by the NIP were very low, less than 3.7% in all cases. A spinach extract showed a reduction in chlorophyll absorbance to as low as 0.092, which is considered acceptable for quantitative analysis. To the naked eye, the green characteristic color of several plant extracts virtually disappeared after exposure to the chlorophyll MIP.
Zirconia on Silica
Supelco, a division of Sigma Aldrich, has introduced three sorbents for the sample cleanup of complex matrices by removing more fat and color from sample extracts than traditional dSPE phases for QuEChERS methods. Supleco's SupelQuE Z-Sep sorbent is recommended for samples containing hydrophobic analytes in fatty matrices. SupelQuE Z-Sep+ is for samples containing greater than 15% fat, and SupelQuE Z-Sep/C18 is for samples containing less than 15% fat. Figure 3 presents a bar graph for the relative retention of the zirconia on silica products compared to other phases typically used in SPE. For the experiment, a 25-mg portion of each sorbent was used for a 1-mL solution of mono-, di-, and triglycerides in 90:10 (v/v) acetonitrile–water. By eliminating problematic matrix interferences, these new sorbents provide more robust LC–MS methods. This proprietary technology can replace both C18 and primary–secondary amine in current methods without additional method development. Some published applications of these new sorbents showed a decreased presence of fat in the studies of organic contaminants in catfish (24) and in the analysis of 100 veterinary drugs in bovine muscle (25).
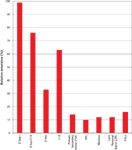
Figure 3: Relative retention of mono-, di-, and tri-glycerides on various sorbents. Courtesy of Sigma-Aldrich, Supelco.
Nanomaterials for Use in Sample Preparation
Nanotechnology is one of the most important trends in material science. Because of their ultrasmall size, nanomaterials possess unique physical and chemical properties. Thus, it is no surprise that nanomaterials have found their way into sample preparation applications. A nanomaterial is used to denote structures that have one of their dimensions in the nanometer size range, 1–100 nm (26). A recent review article discusses the use of nanomaterials in sample preparation (27). So, only brief coverage of the technologies used in sample preparation will be covered here. The so-called nanoscale effect comes into play with particles in the nano-range because they display optical, electrical, thermal, magnetic, catalytic, and other effects that non-nanomaterials do not display. Their larger surface areas allow smaller amounts to be used for extraction and make them ideal for miniaturization of sample preparation protocols. Many nanomaterials can be functionalized with various chemical groups to increase their affinity toward target compounds. A wide variety of nanomaterials are being studied for sample preparation. Metallic and metallic-oxide nanoparticles are on the forefront of research in chromatography and sample preparation applications. Gold nanoparticles have been modified with organic molecules with thiol or amino groups to form self-assembled monolayers. Derivatized nanoparticles can be used as coatings for SPME by assembling them on a stainless steel wire, which then can be used to extract polynuclear aromatic hydrocarbons from water. Silver nanoparticles have also been used for SPME. Metal oxide nanoparticles such as TiO2, ZrO2, and Al2O3 have been widely used for extraction and separation of pollutants both as SPE sorbents and as coatings for SPME. When coated onto SPME fused-silica fibers or rods, compared to commercial phases, new selectivities can be introduced and the SPME devices are often more rugged. The metal oxide nanoparticles can also be further functionalized with small organic or inorganic compounds or polymers.
Among the more interesting nanoparticles are those that are based on magnetic adsorption materials. The materials can be used in a dSPE mode. These materials are easily manipulated via magnets and can save time compared to using SPE manifolds or centrifuges for separating the solid phase from the supernatant liquid. SPE columns do not need to be packed and since no liquid sample loading of a cartridge is required, the experiment is much faster. Typically, the magnetic material has an iron oxide core and a coating of nanoparticles to extract different analytes. In a paper by Tian and colleagues (27), a table of various applications of magnetic nanoparticles is shown.
Other types of nanoparticles that have been used include metal–organic framework materials. These are organic–inorganic hybrids and can be fashioned with different metallic cations and different organic electron donor linkers to provide derivatizable surfaces, which can be used for many different applications including sample preparation such as SPE and SPME. They can also be magnetized for easy handling.
Many people are familiar with carbonaceous nanomaterials such as carbon nanotubes, fullerenes (buckyballs), and carbon nanocones. Graphene is the basic building block of carbon sorbents such as graphitic carbon black, which was mentioned earlier. Graphene powder has been used as an SPE sorbent for the extraction of chlorophenols from water but, as mentioned earlier, it has a tendency to aggregate so immobilizing it on a substrate such as silica gel or converting it into graphene oxide can make it more useful for sample preparation. It has also been used in SPME. Carbon nanotubes are the hottest carbon nanostructure material. Because of their unique geometry, carbon nanotubes exhibit excellent mechanical and thermal properties. Carbon nanotubes can be functionalized and used in SPE and SPME. There are many publications on the use of carbon nanotubes in sample preparation (27).
Silica nanoparticles are most similar to those used in regular SPE experiments including the incorporation of molecular imprinting to provide high affinity and selectivity to the nanoparticles compared to normal C18 and other common groups.
Nanomaterials should continue to see rapid development, especially as further miniaturization takes place in chromatography and sample preparation.
New Sorbent Materials
New sorbent materials are continually coming out of the research laboratories, some of which may prove to be the next universal sorbent or be the most selective ever. A couple of examples are shown below.
Mixed-Mode Sorbents
In the past, mixed-mode behavior of sorbents was considered to be a nuisance because it was harder to use one's chromatographic knowledge to develop a method. Workers in the field preferred pure reversed-phase mechanisms so one could predict how the change in organic modifier or pH would affect retention. Nevertheless, mixed mechanisms still occurred that often complicated things. Nowadays, mixed mechanisms are now looked upon as good, especially in sample preparation, because one can now manipulate the conditions to vary the relative interactions between the analyte, matrix, and sorbent to come up with the best set of conditions to isolate the analyte of interest. Manufacturers are now introducing SPE phases with dual- or even trifunctionality. For example, Biotage has introduced several SPE phases that provide both nonpolar and ion-exchange functionalities that can be used to remove interferences that would potentially cause ion suppression or enhancement in LC–MS-MS analysis. The products are resin-based, but silica-based mixed-mode sorbents are also available to perform the same type of extractions.
Moral and colleagues from Spain (28) synthesized their own multifunctional sorbents based on sodium dodecyl sulfate (SDS) and mixed tetrabutylammonium (TBA)-SDS hemimicelles and micelles adsorbed onto gamma-alumina. These sorbents provided different retention mechanisms (such as hydrophobic, ionic, and π-cation interactions). They used these mixed-mode sorbents for the extraction of acidic, basic, and neutral pesticide multiresidues from natural waters. Recoveries ranged between 92% and 107% for most of the pesticides tested. Using UV detection, the workers had detection limits lower than 100 ng/L for these pesticides.
Fontanals and colleagues (29) synthesized a copolymer based on N-vinylimidazole–divinylbenzene that showed mixed-mode and both weak and strong anion-exchange properties. The SPE copolymer could be protonated at a certain pH and used as an anion exchanger, and at other pH values its hydrophilic and reversed-phase properties could be exploited. The novel copolymer was compared to commercially available polymeric SPE sorbents and showed unique selectivity.
Marshmallow Silicone Monoliths
At HPLC 2013, Kazuki Nakanishi and coworkers from Kyoto University and GL Science in Japan (30) reported on recent developments in a macroporous poly(methylsilsesquioxane) (PMSQ) monolith family that produced a novel type of siloxane-based macroporous polydimethylsiloxane (PDMS) analogs suitable for many kinds of sample preparation purposes. These so-called "marshmallow gels" are very soft, low density, highly hydrophobic, and highly permeable monoliths with micrometer-range continuous pores and are synthesized by controlled copolymerization of methyltrimethoxysilane and dimethyldimethoxysilane (MTMS-DMDMS) precursors. Because of the presence of dense surface methyl groups, all the surfaces of micrometer-range skeletons are highly hydrophobic and exhibit a similar or faster equilibration rate than those of other PDMS-based SPE devices. Physical properties are comparable to conventional PDMS materials; marshmallow gels exhibit no degradation under temperatures as high as 600 K, and no obvious glass transition at temperatures as low as 150 K. Furthermore, even under liquid nitrogen (77 K) the material still exhibits rubbery elastic deformation and recovery. In the above broad temperature range, the extraction of hydrophobic substances from a polar solvent system can be dramatically accelerated by absorbing the mixture and simply squeezing it out. These gels may find use in both in GC and LC sample preparation purposes. Not limited to the PDMS analog, the chemical modification of the pore surface is easy by choosing appropriate precursors. Typical processing requires only a few hours in any shape and size below 100 °C. The use of this unique polymer for SPE is depicted in Figure 3. Because the sorbent is selective for hydrophobic compounds, pyrene in water was selected as a test solute. In Figure 4, only 10 μL of the test sample (9.57 mg/20 mL acetonitrile) was added to 100 mL of water and the pyrene recovery was 98.2%; using HPLC with UV detection, the pyrene showed good linearity over the 0.05–5 mg/L concentration range.
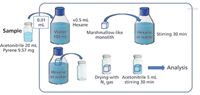
Figure 4: Application of marshmallow silicone monolith in solid-phase extraction. (Courtesy of Gen Hayase, Kyoto University [28].)
Conclusions
In this installment, I tried to select some significant new approaches to sorbent development that might results in better, faster, more efficient, and cleaner sample preparation than previous sorbent materials used in the general category of solid–liquid extraction. Further development in MIPs seems especially strong in Europe with new developments in phases, format (that is, stir bars), and preparation or template removal techniques. The dSPE portion of the QuEChERS extraction has been getting attention as workers try to come up with better sorbents to selectively remove matrix compounds that may interfere with downstream LC–MS-MS and GC–MS-MS analysis. Nanoparticles have attracted lots of attention but few commercial products are currently available. Nevertheless, the future looks particularly bright as smaller sample sizes and miniaturization of sample preparation advances further. New sorbent materials continue to be introduced for solid–liquid sample preparation, and this trend should continue in both the academic and commercial sectors.
References
(1) K. Ensing, C. Berggren, and R.E. Majors, LCGC North Am. 19(9), 942–954 (2001).
(2) S.G. Dmitrienko, V.V. Irkha, A. Yu. Kuznetsova, and Yu. A. Zolotov, J. Anal. Chem. 59(9), 808–817 (2005).
(3) C. Baggiani, L. Anfossi, and C. Giovannoli, Current Pharma. Anal. 2(3), 219–247 (2006).
(4) J.O. Mahony, K. Nolan, M.R. Smyth, and B. Mizaikoff, Anal. Chim. Acta 534, 31–39 (2005).
(5) P.A.G. Cormack and A.Z. Elorza, J. Chromatogr. B 804, 173–182 (2004).
(6) S. Piletsky and A. Turner, Molecular Imprinting of Polymers (Landes Bioscience, Austin, Texas, 2006), p. 220.
(7) M. Komiyama, T. Takeuchi, T. Mukawa and H. Asanuma, Molecular Imprinting: From Fundamentals to Applications (Wiley-VCH, 2003), p. 159.
(8) L. Ye, Molecular Imprinting: Principles and Applications of Micro- and Nanostructure Polymers (Pan Stanford Publishing, 2013), p. 290.
(9) C.I. Lin, A.K. Joseph, C.K. Chang, Y.C. Wang, and Y.D. Lee, Anal. Chim. Acta 481, 175–180 (2003).
(10) H. Liu, K.H. Row, and G. Yang, Chromatographia 61, 429–432 (2005).
(11) B. Sellergren, J. Chromatogr. A 673, 133–141 (1994).
(12) H. Yan, F. Qiao, and H. Ho Row, Chromatographia 70(7–8), 1087–1093 (2009).
(13) J.Matsui, M. Okada, M. Tsuruoka, and T. Takeuchi, Anal. Commun. 34, 85–87 (1997).
(14) C. Chen, L. Yang, and J. Zhou, J. Applied Polymer Sci. 122, 1198–1205 (2011).
(15) X. Zhu and Q. Zhu, J. Applied Polymer Sci. 109, 2665–2670 (2008).
(16) A. Martin-Esteban and E. Turiel, J. Separation Sci. 35, 2962–2969 (2012).
(17) B.S. Batlokwa, J. Mokgadi, T. Nyojong, and N. Torto, Chromatographia 73, 589–593 (2011).
(18) R.E. Majors, LCGC North Am. 31(11), 914–924 (2013).
(19) R.E. Majors, LCGC North Am. 25(5), 436–446 (2007).
(20) S.J. Lehotay, M. Anastassiades, and R.E. Majors, LCGC North Am. 28(7), 504–516 (2010).
(21) L. Zhao and J. Stevens, "Optimizing Recoveries of Planar Pesticides in Spinach Using Toluene and Agilent Bond Elut QuEChERS AOAC Kits with Graphitized Carbon," Agilent Application Note #5990-4247EN, 2012.
(22) D. Stoll, D.C. Harmers, J. Thompson, D. Fryer, C. Smith, J. Stevens, and B. Barber, "Novel Porous Carbon Sorbent Materials for Use in Sample Preparation," presented at HPLC 2013, Amsterdam, The Netherlands, 2013.
(23) B.S. Batlokwa, J. Mokgadi, R.E. Majors, C. Turner, and N. Torto, J. Chem. ID 540240 (2013).
(24) Y. Sapozhnikova and S.J. Lehotay. Anal. Chim. Acta 758, 80–92 (2013).
(25) L. Geis-Asteggiante, S.J. Lehotay, A.R. Lightfield, T. Dutko, C. Ng, and L. Bluhm, J. Chromatogr A 1258, 43–54. (2012).
(26) R.J. White, R. Luque, V.L. Budarin, J.H. Clark, and D.J. Macquarrie, Chem. Soc. Rev. 38, 481 (2009).
(27) J. Tian, J. Xu, F. Zhu, T. Lu, C. Su, and G. Ouyang, J. Chromatogr. A 1300, 2–16 (2103).
(28) A. Moral, M.D. Sicilia, S. Rubio, and D. Perez-Bendito, Anal. Chim. Acta 608, 61–72 (2008).
(29) N. Fontanals, B.C. Trammel, M. Galia, R.M. Marce, P.C. Iraneta, F. Borrul, and U.D. Neue, J. Sep.Sci. 29, 1622–1629 (2006).
(30) G. Hayase, K. Morisato, K. Kanamori, and K. Nakanishi, "A New Stationary Phase for Solid-Phase Extraction with Marshmallow-like Silcone Monoliths," presented at HPLC 2013, Amsterdam, The Netherlands, 2013.
Ronald E. Majors "Sample Prep Perspectives" Editor Ronald E. Majors is an analytical consultant and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives," LCGC, Woodbridge Corporate Plaza, 485F Route 1 South, Suite 210, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com.

Ronald E. Majors


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)