Novel Sample Preparation Methods for Analyzing Pain Management Drugs in Complex Matrices
Despite the complex matrices present in toxicology testing, fast, accurate results should be possible with easy-to-implement sample preparation and liquid chromatography with tandem mass spectrometry (LC–MS/MS) analysis. Often, to prepare a diverse sample set, the quickest method may have unintended negative impacts. Sample preparation methods need to be selected based on the pain management drugs being analyzed, cleaning up the matrix without compromising recovery, column lifetime, or to reduce additional system maintenance in the future. This article will explore the same 39 analytes in urine, whole blood, and plasma as well as the differences between the workflows to get consistent, fast MS results.
Traditional pain management drug testing poses many analytical challenges for laboratories, including the use of sample preparation techniques that are often time‑consuming and unreliable. In addition, a wide variety of sample matrices exist and can potentially provide inaccurate results. Each of these matrices present different challenges that could complicate switching an assay or updating validated methods due to the specific clean‑up requirements. Whole blood, serum, and urine are common matrices that all require sample preparation before liquid chromatography tandem mass spectrometry (LC–MS/MS) analysis, yet laboratories struggle to find reproducible, accurate, and reliable workflows. While the preparation of biological samples has not changed significantly in the past 10 years, different techniques have evolved to process more samples in less time while also yielding more sensitive results, all due to advancements in instruments. The implementation of simple sample preparation methods before selecting an LC column is an important step towards a consistent, efficient workflow for analyzing pain management drugs.
The first step for developing a new method is to evaluate the matrix, as well as what level of sensitivity is needed for the assay. This can differ between toxicology laboratories based on their needs and the type of analytes they are working to separate. Most laboratories have great resources for optimizing or developing methods from chromatography vendors. However, understanding method requirements will help in implementing a cohesive, dependable method. To get a robust sample preparation method, analysts should select a sample preparation technique based on the starting matrix and then proceed to evaluate an LC column. Sample preparation is usually an afterthought, even though it could directly impact the LC separation, resolution, matrix effects, and more. While most laboratories are not establishing completely new methods or assays, the need for laboratories to be able to evolve to meet specific analyte levels must be considered; this requires an understanding of selecting sample preparation methods based on diverse matrices, as well as a variety of analytes with different chemical structures.
Urine Cleanup
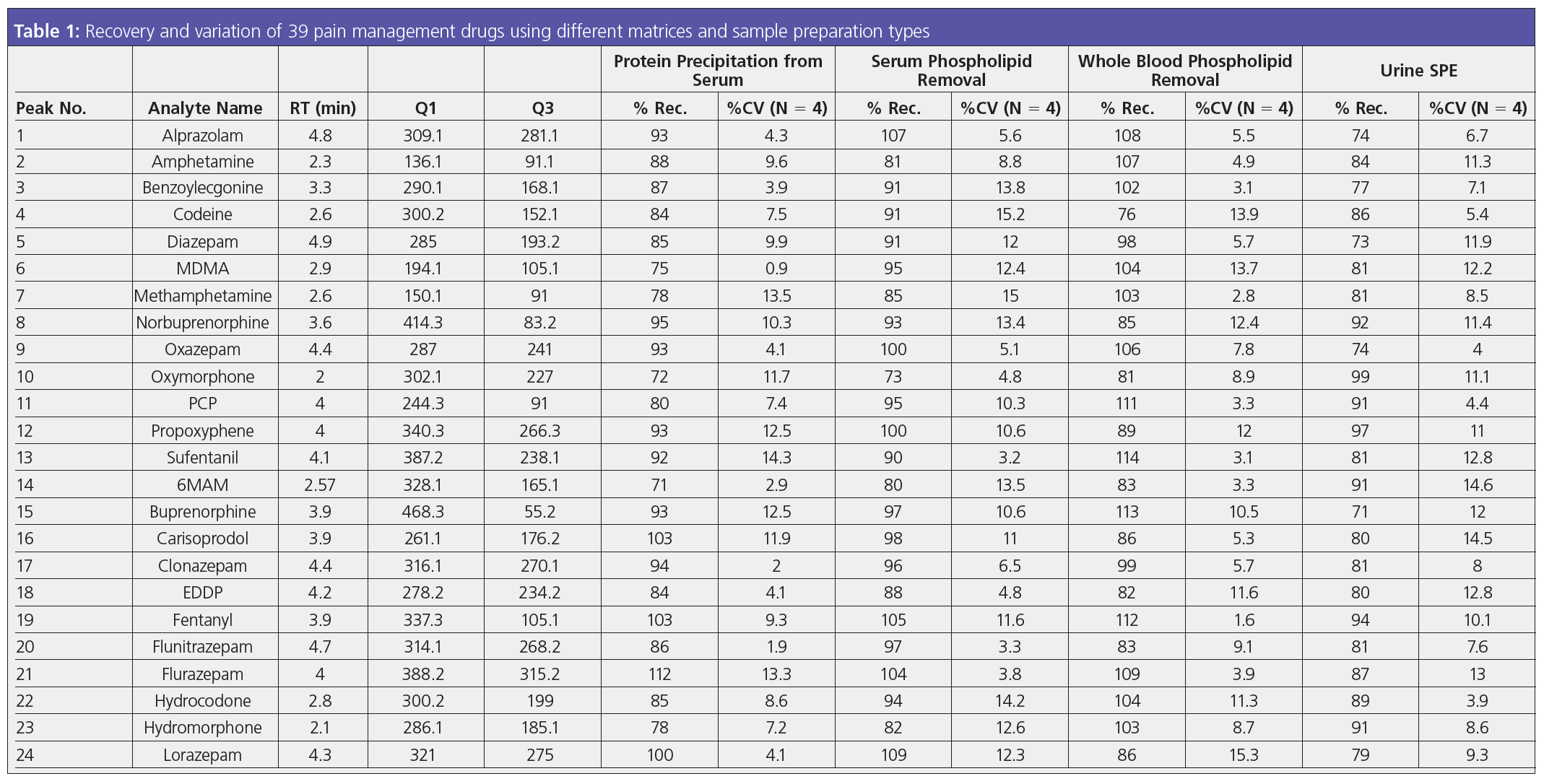
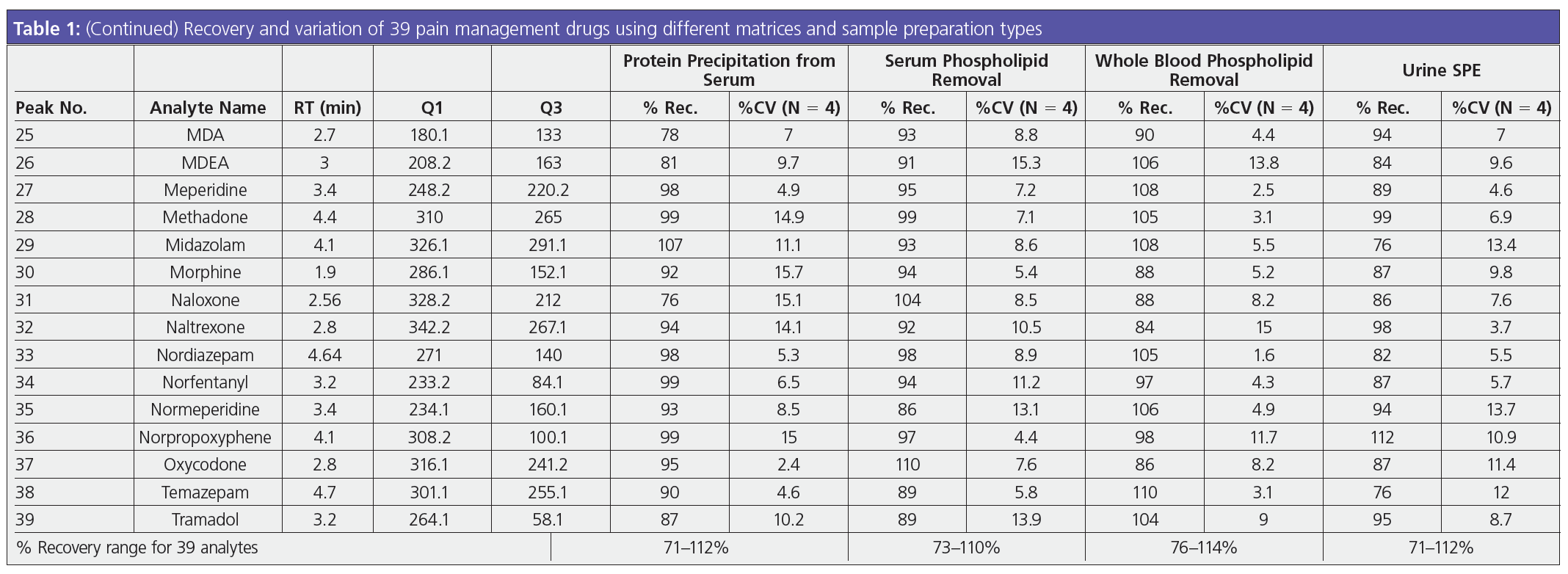
As a sample matrix, urine seems relatively straightforward. However, the clean‑up techniques may not be. A universal drug test is often performed using urine, which is thought of as an easy matrix to work with. While there are many clean‑up techniques, filtration, dilutions, and solid‑phase extraction (SPE) are some of the most frequently used. To determine the most appropriate method, analysts and method developers need to weigh the cleanliness, costs, and results. One of the downsides of testing urine is that drugs have already been metabolized—meaning they have undergone a glucuronidase reaction in the liver to increase polarity so the drug moiety can be absorbed into the kidneys (1). The result is a drug structure that must be hydrolyzed to be analyzed by LC–MS/MS. After hydrolysis, the drug as well as a large protein are present in the sample. For some laboratories, a dilution is a simple solution. However, the trade-off of this method is that LC columns will fail prematurely, affecting the reliability of results and causing more expenses for the laboratory down the line. By using filtration, some of the large proteins and particulates can be removed prior to analysis while maintaining the sensitivity in the sample that may be required downtream. SPE is a selective sample preparation technique that results in the cleanest extraction, but it can take a significant amount of method development and time. An SPE method that does not require a conditioning or equilibration step—about 40% of the protocol—allows analysts to achieve the benefits of SPE in less time, with high recoveries and low variation between samples (2). Table 1 shows the effectiveness of a method based around drug extractions to save method development time but still yield accurate, high recoveries.
Plasma and Serum Cleanup
For drug analysis, plasma and serum allow for better real-time detection data then urine. Plasma and serum act as good indicators, providing accurate LC results. However, the sample is more complex than urine; a simple dilution will not effectively clean up the sample for LC–MS/MS. These types of matrix require either a protein precipitation, phospholipid removal, or SPE prior to analysis. Spending time developing an LC method with the correct selectivity and separation will be ineffective if the proteins and phospholipids in the samples are not removed prior. The phospholipids present in the sample can cause ion suppression or enhancements in the MS system at specific transitions, introducing further inaccuracies and a change in the baseline. Figure 1 shows an analysis that was performed to determine 39 pain management analytes in urine and the method is detailed below.
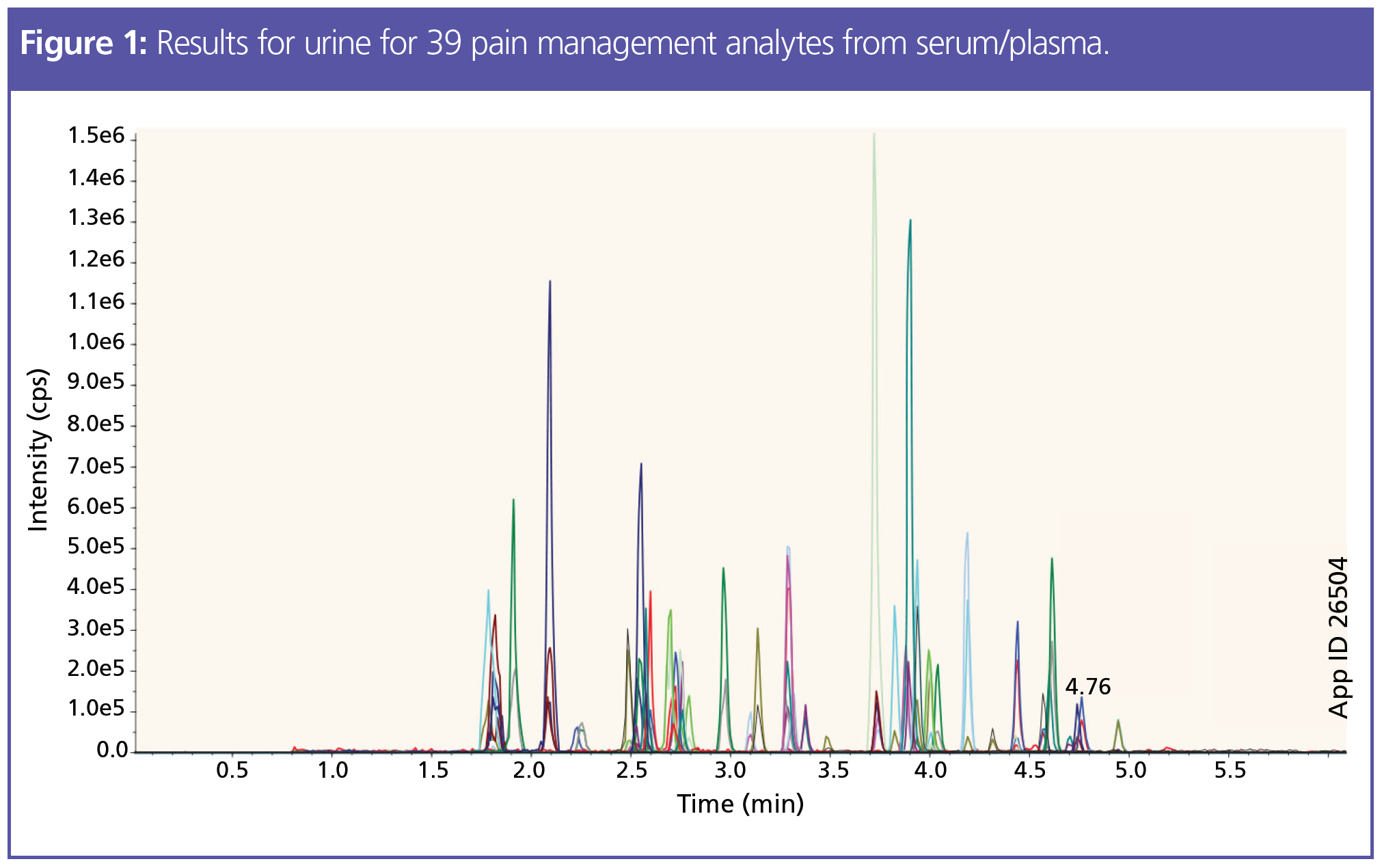
Experimental: LC–MS/MS: Column: 50 × 3.0 mm, 2.6-µm Kinetex Biphenyl; (Phenomenex) part no.: 00B-4622-Y0; mobile phase: A: 0.1% formic acid in water, B: 0.1% formic acid in methanol; gradient: 0 min 15% B, 3.5 min 95% B, 5 min 95% B, 5.01 min 15% B, 7 min 15% B; flow rate: 0.5 mL/min; injection volume: 5 µL; temperature: ambient; detection: Sciex 4500 MS/MS (ESI+)
Sample Preparation: 200 µL of serum was aliquoted into a tube and 600 µL of chilled (0 to -20 ºC) 95:5 acetonitrile–methanol was added and vortexed for 5–10 s. The tube was centrifuged at 300 rpm for 10 min, the supernatant was collected, and 25 µL of 1% formic acid was added. Load: pre-treated sample onto the Phree 96‑well plate; part no.: 8E-S133-TGB; vacuum: 4–5 psi to collect supernatant; dry down: sample under a gentle stream of nitrogen at 40–45 ºC; reconstitute: in 200 µL initial mobile phase. The difference in the chromatography between samples that had undergone a simple protein precipitation and those which used phospholipid removal is shown in Figure 2. Phospholipid removal is one of the easiest sample preparation methods to implement and balances time, recovery, and matrix cleanliness. It does not require method development—the time spent is equivalent to protein precipitation—and it can be easily automated for high‑throughput samples. Cleanup can also be done using a solid‑phase extraction method if additional solvent switching or concentration are concerns, but the time spent on method development and the protocol itself will be increased in this case. By using phospholipid removal, the phospholipid profile will be significantly cleaned up, leading to better MS results, longer column lifetimes, lower backpressure, and overall improvement in the chromatography (3). The recovery results of the extraction still remain high, with a low percentage of variation as seen in Table 1.
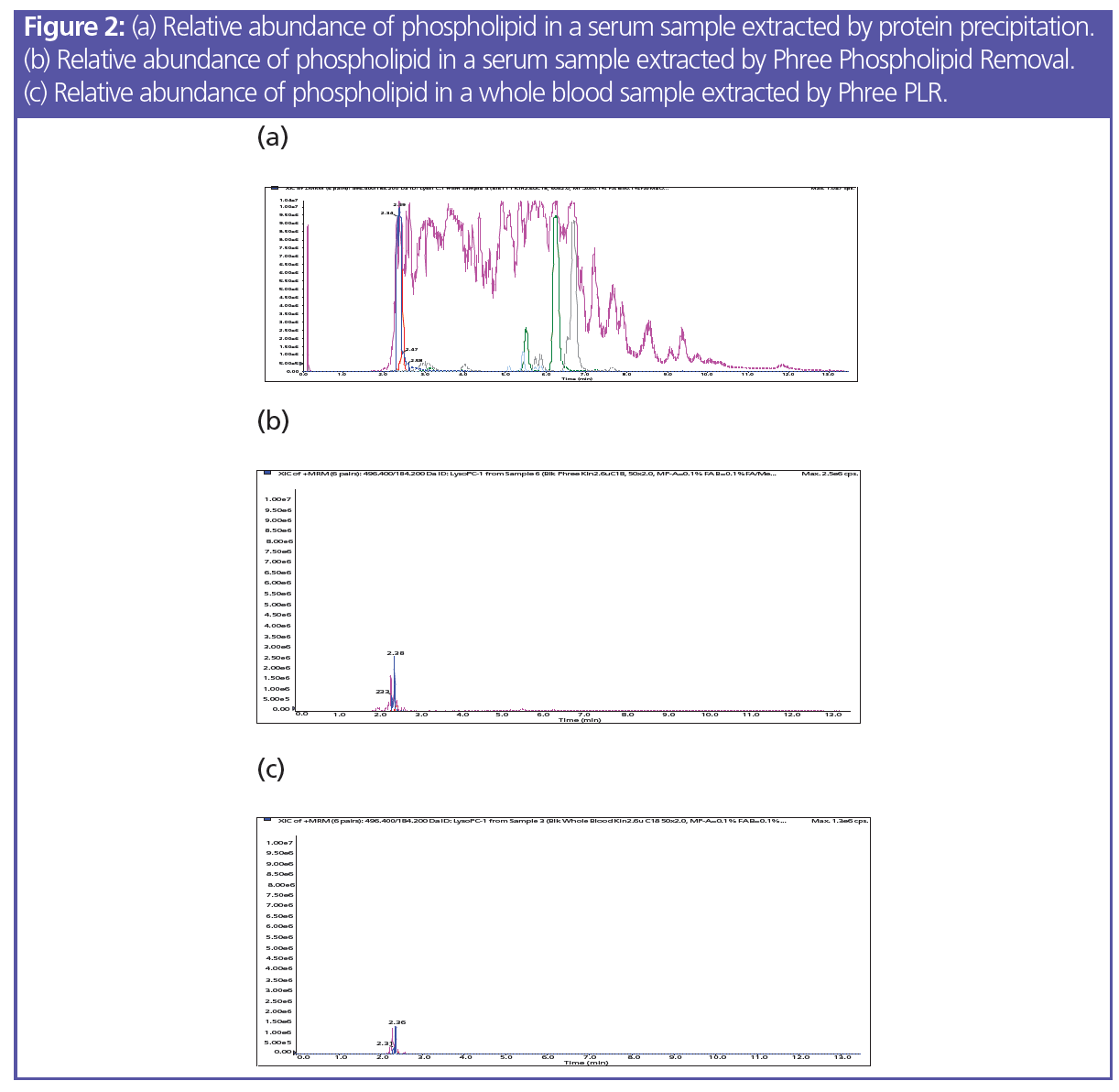
Whole Blood Cleanup
Whole blood as a sample matrix is commonly used within toxicology testing laboratories. Like plasma or serum and unlike urine, the drugs present in whole blood have not yet been metabolized, so the hydrolysis step can be skipped. However, whole blood as a matrix has disadvantages, including the complexity and the required pretreatment steps needed even before preparing the appropriate sample. Whole blood must be broken down; phospholipid removal or solid-phase extraction is required for clean up. SPE is the best solution for laboratories that need to add sensitivity at low levels. When working with a diverse panel of drug analytes, it is imperative to have a solution unique to the matrix and drug panel. While many SPE sorbents and tube formats are available, the basic chemical reaction remains the same: the tube is primed by the conditioning and equilibration steps, the sample is loaded, solvents are used to wash the tube, analytes are bonded to the sorbent, and a strong organic solvent is used to “release” the analytes. Then they are ready for LC separation for identification. Phospholipid removal also works similarly after the whole blood pretreatment to serum or plasma; if a laboratory needs a relatively fast, clean sample, they typically implement phospholipid removal techniques and subsequently improve the chromatography (4). Results for the two-step phospholipid removal are seen in Table 1. The results are comparable to what you would get with an SPE method for whole blood.
Liquid Chromatography (LC) Column Selection
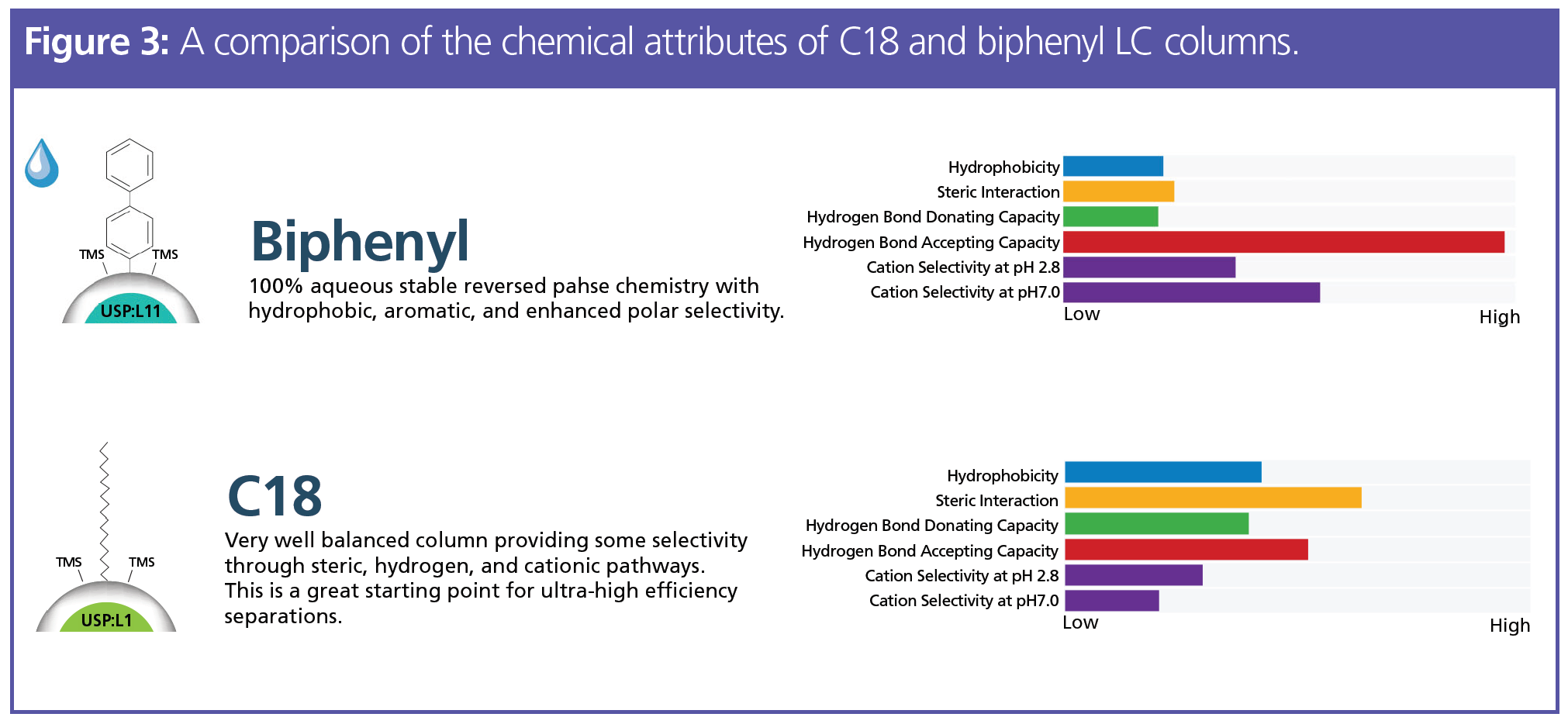
Selecting an LC column for the separation and analysis of drug analytes is a more straightforward approach than sample preparation. Additionally, troubleshooting issues with the separation method is typically simpler than troubleshooting the sample preparation method. Method development requires optimizing the stationary phase as well as overall conditions to achieve the separation of target analytes. For the majority of pain panel or pain management testing, a selectivity with 100% aqueous stability is recommended for reversed phase separations. To understand why a biphenyl selectivity is often adopted for different drug assays, a comparison of the chemical attributes on a core–shell backbone is highlighted in Figure 3. Both the C18 and biphenyl LC columns are bonded on a core–shell solid support, which offers an efficient way to potentially increase relative peak intensity while operating under typical high performance liquid chromatography (HPLC) pressure (<400 bar). An example of the separation for 39 different pain management drugs using a biphenyl bonded on a core–shell LC particle, with the sample preparation method from serum and with good chromatography results, is shown in Figure 1. In the future, there will be MS instruments that guarantee analyte detection at lower levels and that incorporate the advantages of micro- and nano-LC technology. With these advances, sample preparation will become an even more important part of the workflow. Nano- and micro‑LC columns cannot be used without appropriate sample preparation, making technique selection even more critical. As previously mentioned, dilution is a traditional method for more sensitive MS instruments, but will not be a viable option with low flow chromatography.
With new specifications, drug moieties, and detection limits, sample preparation methods need to be adaptable for laboratories that work in a fast-paced separation environment. When working with a diverse array of matrices for different pain management extraction, each type of matrix provides a slew of options for sample preparation. Since not all samples are uniform, neither are the sample preparation options. However, laboratories should consider how to balance clean-up techniques with their scheduling and budgetary requirements.
References
- J. Trontelj, Quantification of glucuronide metabolites in biological matrices by LC-MS/MS, tandem mass spectrometry—applications and principles, In J. Prasain, Ed., InTech (2012). www.intechopen.com/books/tandem-mass-spectrometry-applications-andprinciples/quantification-of-glucuronide-metabolites-in-biological-matrices-by-lc-ms-ms
- Z. Cao, E. Kaleta, and P. Wang, J. Anal. Toxicol. 39(5), 335–46 (2015).
- S. Kushon and E. Pike, Current Trends in Mass Spectrometry 10(4), 28–31 (2012).
- S. Huq, Fast Clean Up of Whole Blood Using Phree Phospholipid Removal and Analysis of 39 Pain Management Drugs by Kinetex 2.6 µm Biphenyl (AN-1018).
Jenny Cybulski graduated from Vanguard University of Southern California (California, USA) with a B.S. in biology and a minor in chemistry. She now works as the Senior Manager of the Global Product Marketing Team at Phenomenex, Inc. located in Torrance, California, USA.
Bryan Tackett graduated from Baylor College of Medicine (Texas, USA) with a Ph.D. in translational biology and molecular medicine. He is the Technical Marketing Writer at Phenomenex, Inc. located in Torrance, California, USA.
Shahana Huq is a senior scientist within the applications lab at Phenomenex. She holds a MS in biochemistry from California State University, Los Angeles, USA. Her work involves analytical method development, optimization, and troubleshooting.
E-mail: JennyCy@phenomenex.com
Website: www.phenomenex.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.
















