New HPLC, MS, and CDS Products Introduced in 2021–2022: A Review
This instalment describes newly introduced high performance liquid chromatography (HPLC), mass spectrometry (MS), chromatography data systems (CDS), and related products in 2021–2022 and prior years. It summarizes their unique features and significant user benefits.
The 74th Pittcon was supposed to be held at Georgia World Congress Center in Atlanta, USA, from 5–9 March 2022. Unfortunately, the global conference was cancelled in mid-January due to the continuing surge of the omicron variant in America. The abrupt cancellation resulted in a limited virtual technical programme without any exposition or short courses. Therefore, the product information collected here is based solely on manufacturers’ websites, returns from the annual LCGC survey, and direct communication from my personal contacts.
Current Product Trends of HPLC and MS Instruments
The current high performance liquid chromatography (HPLC) and mass spectrometry (MS) instrument portfolios utilize mature technologies that have benefitted from decades of continuous product improvements.
The advent of ultrahigh-pressure liquid chromatography (UHPLC) and its first product commercialization in 2004 (1) had brought on a surge of instrumental redesigns and a tidal wave of new product offerings by the four leading manufacturers in recent years (Waters, Agilent, Shimadzu, and Thermo Fisher Scientific) (2). The MS instrument market is dominated by the same four big manufacturers, plus Sciex and Bruker (2).
While there were fewer new introductions or significant upgrades of HPLC instruments, manufacturers have added systems with intermediary pressures, dual‑injection, or multidimensional capability. Many expanded their product offerings in preparative purification, micro‑LC, bio-inert, and application‑specific systems in areas such as amino acid analysis, cannabis assays, two-dimensional liquid chromatography (2D-LC), method development, and clinical diagnostics (3–5).
Similar paths were followed for MS instrumentation, with trends towards more compact single quadrupole (SQ) or triple quadrupole (TQ) MS, ion mobility MS (IMS), direct sampling of solids and systems focused on specific applications such as multi-attribute biopharmaceutical analysis, clinical diagnostics, and biological imaging (2–5).
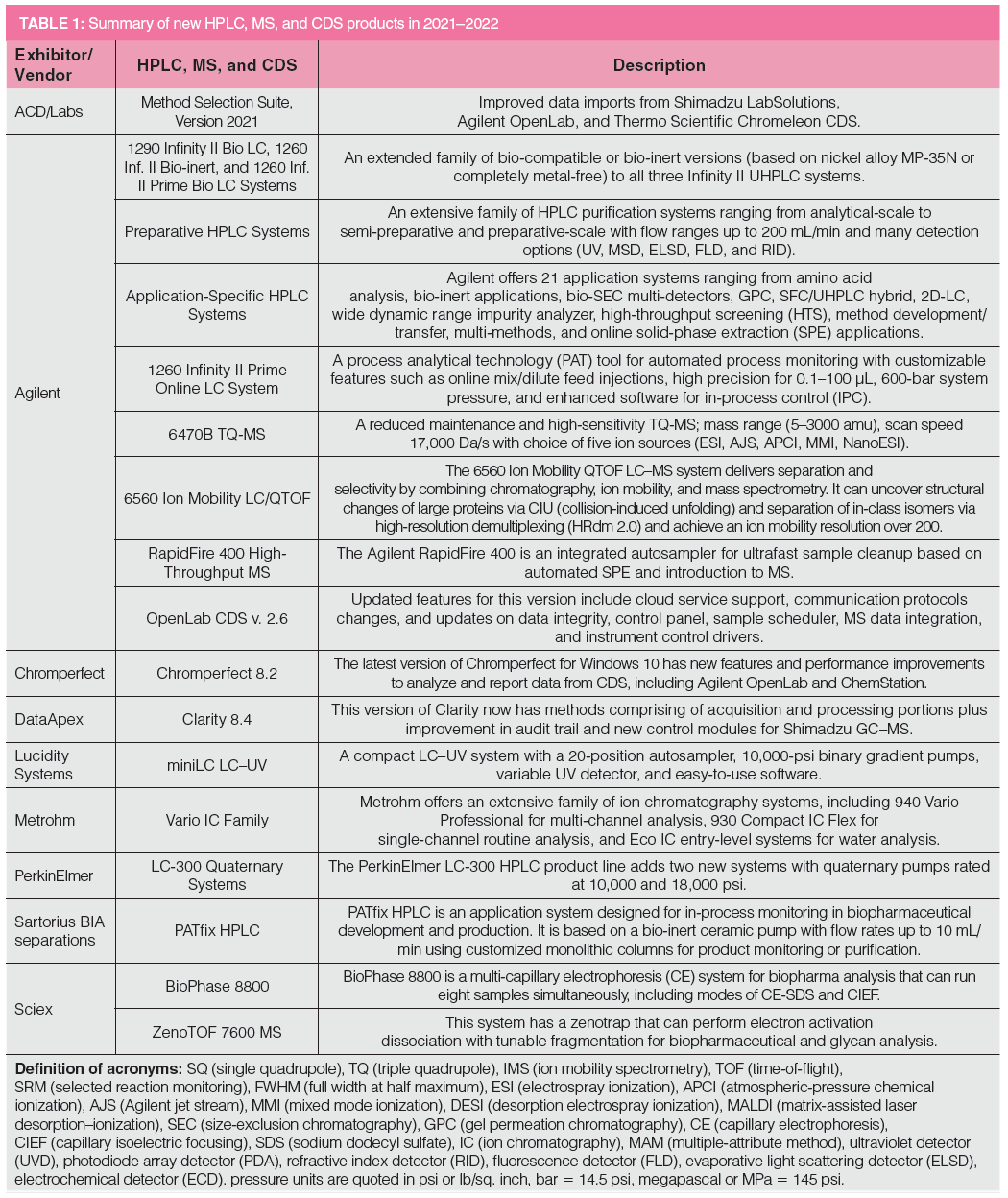
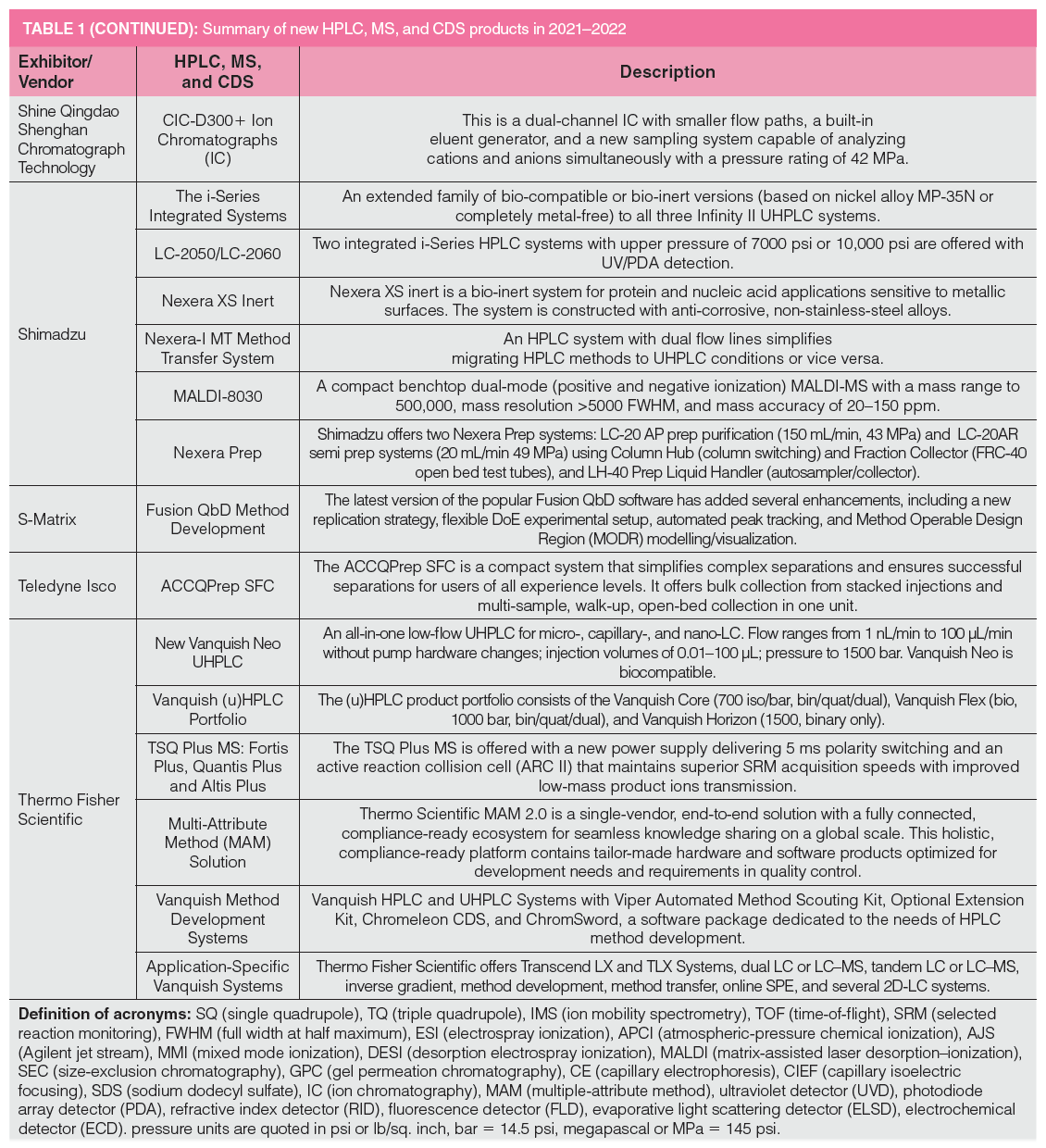
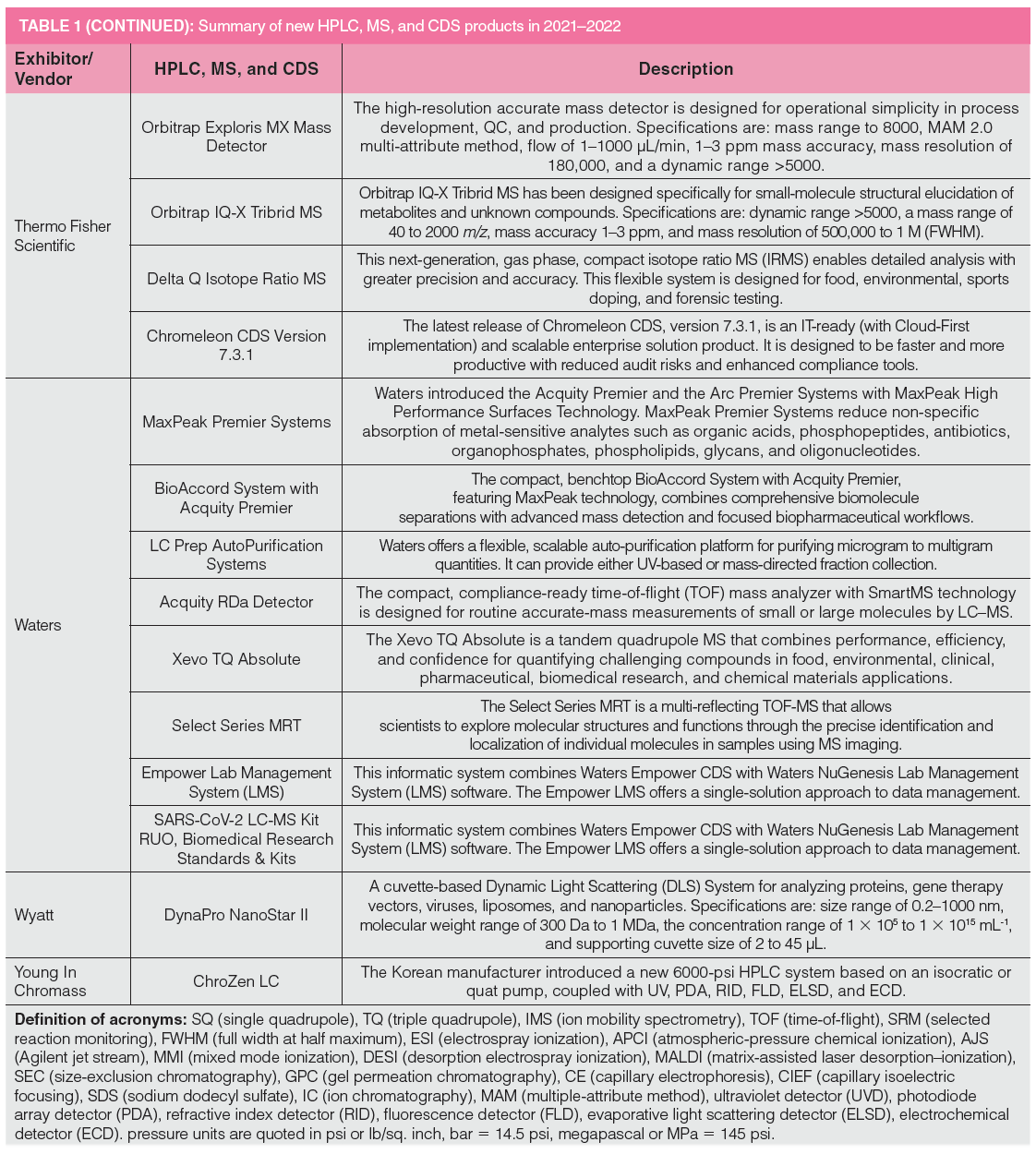
Table 1 lists new product introductions ordered alphabetically by the vendor’s name from 2021 to 2022, categorized as HPLC systems, modules, MS, chromatography data systems (CDS), and other software. The HPLC system category includes ion chromatography (IC) and supercritical fluid chromatography (SFC) instruments. Key innovative features and benefits of each product are briefly described later. Several products or upgrades introduced before 2021 are included here since they were not covered in previous instalments. The footnotes of Table 1 contain the definitions of common acronyms used later in the text.
HPLC/UHPLC Systems (including IC and CE)
The introduction of new UHPLC systems has slowed as manufacturers focused more on HPLC line extensions for higher productivity, ease of use, and specific applications such as 2D‑LC, biopharmaceutical multi-attribute methods (MAM), cannabis analysis, and clinical diagnostics (2–5). The followings are brief descriptions of those products with significant impacts. The reader is referred to manufacturers’ websites and product brochures for more details and specifications.
Agilent Infinity II Bio LC Systems: Agilent offers an extended family of bio-inert versions based on a nickel alloy MP-35N for the three Infinity II UHPLC systems (1290, 1260, and 1260 Prime Infinity II). Features available for these systems include Intelligent System Emulation Technology (ISET), Instrument Control Framework (ICF), and LC–MS or size-exclusion chromatography (SEC) capabilities.
Agilent Preparative HPLC Systems: An extensive family of HPLC purification systems based on 1220, 1260, or 1290 Infinity II modules ranging from analytical-scale to semi-preparative and preparative-scale with flow ranges up to 200 mL/min and software towards workflow automation. An extensive range of detection options is available (ultraviolet [UV,], mass spectrometry detection [MSD], evaporative light scattering detection [ELSD], fluorescence detection [FLD], and refractive index detection [RID]).
Agilent Application-Specific HPLC Systems: Agilent offers 21 application systems ranging from amino acid analysis, bio-inert applications, bio-inert SEC multi-detectors, gel permeation chromatography (GPC), supercritical fluid chromatography (SFC)/UHPLC hybrid, 2D-LC, 2D-LC (Bio), wide dynamic range impurity analyzer, high-throughput screening (HTS), method development and transfer, multimethods, and online solid-phase extraction (SPE) applications.
Agilent 1260 Infinity II Prime Online LC System: A process analytical technology (PAT) tool for automated process monitoring with customizable features such as online mix/dilute feed injections, high precision for 0.1–100 µL injection volumes, 600-bar system pressure, and enhanced software for in-process control (IPC).
Lucidity Systems miniLC LC–UV: A compact LC–UV system with a 20-position autosampler, 10,000-psi binary gradient pumps, variable UV detector, and easy-to-use CDS software.
Metrohm Vario IC Family: Metrohm offers an extensive family of IC systems, including the 940 Vario Professional for multi-channel analysis, the 930 Compact IC Flex for single-channel routine analysis, and the Eco IC entry-level systems for water analysis.
PerkinElmer LC-300 HPLC and UHPLC Systems with Quaternary Pumps: PerkinElmer adds two new systems to its LC-300 HPLC product line with quaternary pumps rated at 10,000 or 18,000 psi.
Sartorius BIA Separations PATfix HPLC: PATfix HPLC is designed for in‑process monitoring in biopharmaceutical development and production (gene therapies and vaccines). It is based on a bio-inert ceramic gradient pump with flow rates of up to 10 mL/min coupled with a UV or fluorescence detector. It uses customized monolithic columns for product monitoring or purification. The PATfix software offers a single database of chromatograms for multiple analytical systems with data visualization and report generation capabilities.
Sciex BioPhase 8800: The BioPhase 8800 is a multi-capillary electrophoresis (CE) system for biopharmaceutical analysis that can run eight samples simultaneously, including modes of CE-sodium dodecyl sulfate (SDS) and capillary isoelectric focusing (CIEF).
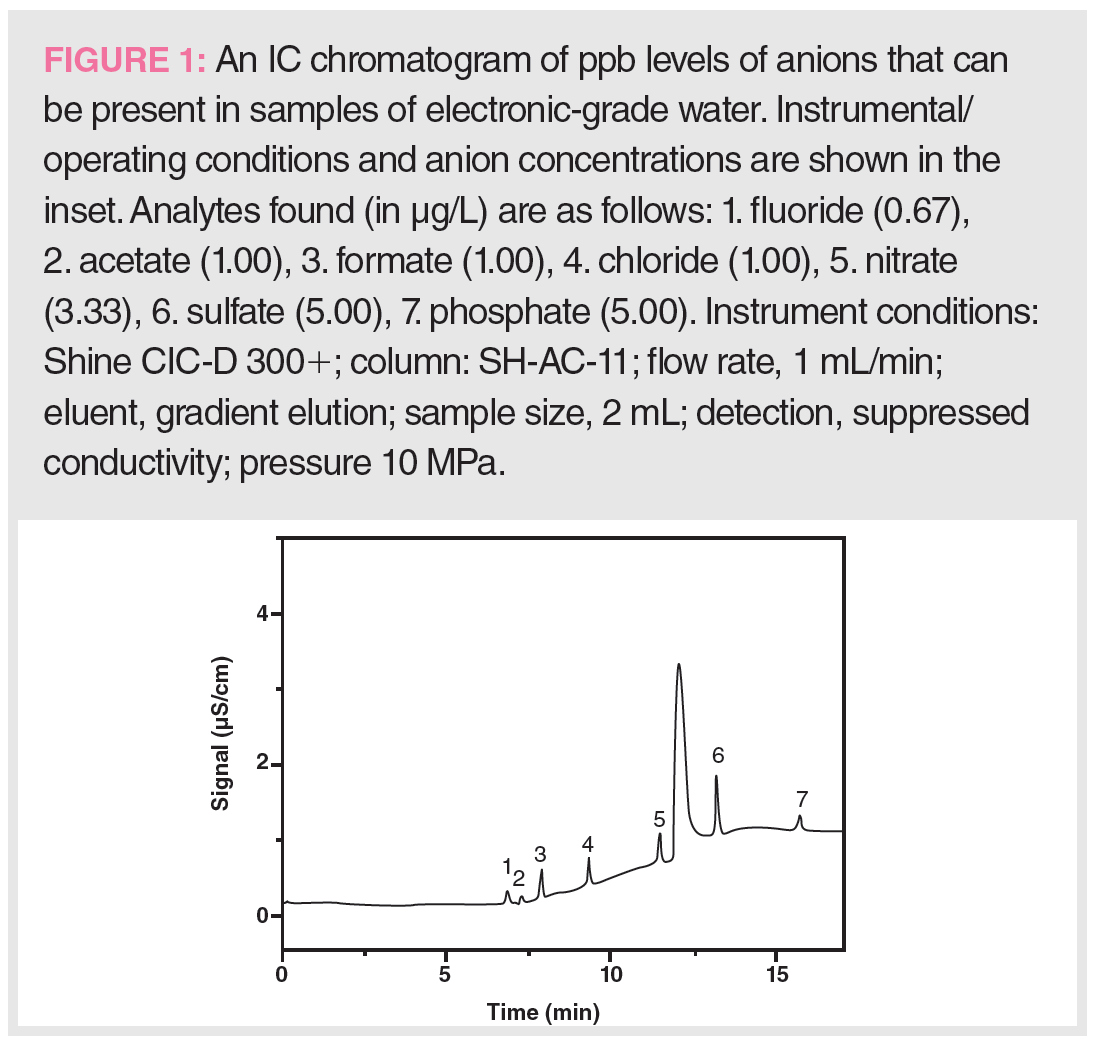
Shine Qingdao Shenghan Chromatograph Technology CIC-D300+ IC: This is a dual-channel IC with a smaller flow path, built-in eluent generator, and a new sampling system capable of analyzing cations and anions simultaneously with a pressure rating of 42 MPa. The improved suppressed conductivity detector delivers a 50% higher sensitivity than its predecessor. Figure 1 shows the sensitivity performance of this new IC in the analysis of common anions in electronic grade water at ppb levels.
Shimadzu i-Series Integrated Systems: Two i-Series HPLC integrated systems based on the LC-2050 or LC-2060 with upper-pressure limits of 7000 psi or 10,000 psi are offered with UV/photo diode array (PDA) detection. Enhancements include a mobile phase monitor, flow pilot, intelligent peak deconvolution, and a simplified user interface. Bio‑inert options are available for high-salt or pH mobile phase operations.
Shimadzu Nexera XS Inert: Nexera XS inert is a bio-inert HPLC system for protein and nucleic acid applications sensitive to metallic surfaces. The system is constructed with anti-corrosive, non‑stainless steel alloys and is based on the new LC-40D XSi solvent delivery unit (up to four pumps can be configured) and a SIL-40C XSi autosampler. System specifications are: pressure rated at 105 MPa; flow range to 3 mL/min, and pH compatibility range of 1–14.
Shimadzu Nexera-i MT Method Transfer System: An HPLC system with dual flow lines simplifies the migration of HPLC methods to UHPLC conditions or vice versa. The system is equipped with Analytical Condition Transfer and Optimization (ACTO) software and the ability to select two delay volumes using a built-in 10-port valve for automated flow path switching.
Shimadzu LabSolutions Method Development System:
The method development system can be configured with modular Nexera or integrated i-Series HPLC/UHPLC systems with automated column/mobile phase screening capability based on the concepts of design of experiments (DoE), predictive simulation, and analytical quality by design (AQbD) optimization algorithms.
Shimadzu Nexera Prep: Shimadzu offers two Nexera Prep systems: LC-20 AP prep purification (150 mL/min, 43 MPa) and LC-20AR semi-prep systems (20 mL/min 49 MPa) using a column hub (column switching), fraction collector (FRC-40 open bed test tubes), and LH-40 Prep Liquid handler (autosampler/collector).
Teledyne Isco ACCQPrep SFC: The ACCQPrep SFC is a compact system that simplifies complex separations and ensures success for users of all experience levels. It offers bulk collection from stacked injections and multi-sample, walk-up, open-bed collection in one unit. The unit has a PDA detector with a gradient pump of 50–300 mL/min flow rate for columns up to 3-cm internal diameter (i.d.).
Thermo Scientific Vanquish Neo UHPLC: An all-in-one low‑flow UHPLC for micro-, capillary-, and nano-LC. Specifications are: flow ranges from 1 nL/min to 100 µL/min without pump hardware changes; injection volumes of 0.01–100 µL; pressure up to 1500 bar.
Thermo Scientific Vanquish HPLC and UHPLC Portfolio: The Vanquish product portfolio consists of the Vanquish Core (700 bar, iso/bin/quat/dual), Vanquish Flex (bio1000 bar, bin/quat/dual), and Vanquish Horizon (1500 bar, binary only). For the entire product portfolio, the injection volume is now extended to up to 1000 µL with larger sample loops of 250 µL or 1000 µL. Vanquish Flex and Horizon are biocompatible.
Thermo Scientific Multi-Attribute Method (MAM) Solution: Thermo Scientific MAM 2.0 is a single-vendor, end-to-end solution with a fully connected, compliance-ready ecosystem for seamless knowledge sharing on a global scale. This holistic, compliance-ready platform contains tailor-made hardware and software products optimized for development needs and requirements in quality control.
Thermo Scientific Vanquish Method Development Systems: Thermo Scientific offers the Vanquish HPLC and UHPLC Systems with Viper Automated Method Scouting Kit, Optional Extension Kit, Chromeleon CDS, and ChromSword, a software package dedicated to the needs of HPLC method development.
Thermo Scientific Application-Specific Vanquish Systems: Thermo Fisher Scientific offers Transcend LX and TLX Systems, dual LC or LC–MS, tandem LC or LC–MS, inverse gradient, method development, method transfer, online SPE, and several 2D-LC application systems.
Waters MaxPeak Premier Systems: Waters introduced the Acquity Premier and the Arc Premier Systems with MaxPeak High Performance Surfaces Technology. MaxPeak Premier Systems reduce nonspecific absorption of metal‑sensitive analytes such as organic acids, phosphopeptides, antibiotics, organophosphates, phospholipids, glycans, and oligonucleotides. The Acquity Premier System is designed for those who need the ultimate in sensitivity, reproducibility, and speed, and provides the best sensitivity and peak shape for analytical separations. The Arc Premier System is designed for those who need to confidently produce accurate, reliable, and reproducible data. It provides robust results from the first injection reliably and quickly.
Waters BioAccord System with Acquity Premier: The compact, benchtop BioAccord System with Acquity Premier, featuring MaxPeak technology, combines comprehensive biomolecule separations with advanced mass detection and focused biopharmaceutical workflows. The Acquity RDa mass detector allows analysts of all abilities to routinely monitor critical quality attributes of biotherapeutics such as intact mass analysis of proteins and oligonucleotides, subunit analysis of monoclonal antibodies (mAbs), peptide mapping/MAM, and released N-glycan analysis, with increased confidence in data quality and system performance.
Waters LC Prep AutoPurification Systems: Waters offers a flexible, scalable auto-purification platform for purifying microgram to multigram quantities based on a high capacity 3767 sample manager coupled with PDA, ELSD, or software quality management systems (SQ MS) with automated fraction collection.
Young In Chromass ChroZen LC:
The Korean manufacturer introduced a new 6000-psi HPLC system based on an isocratic or quaternary pump, coupled with UV, PDA, RID, FLD, ELSD, or electron capture detectors (ECD). Control and data handling are by YL-Clarity CDS. This HPLC system supplements an 18,800‑psi UHPLC system introduced in 2018, which can now be paired with diversified detectors with smaller flow cells.
HPLC Module
Wyatt DynaPro NanoStar II: A cuvette‑based dynamic light scattering (DLS) system for analyzing proteins, gene therapy vectors, viruses, liposomes, and nanoparticles. Specifications are: size range of 0.2–1000 nm, molecular weight range of 300 Da to 1 MDa, the concentration range of 1 × 105 to 1 × 1015 mL-1, and supporting cuvette size of 2 to 45 µL..
Mass Spectrometers (MS)
The marketing landscape for mass spectrometers has been described elsewhere (2–5). Manufacturers continued to upgrade their product offerings in time-of-flight (TOF), triple quadrupole MS, orbital trap, and sample introduction systems to increase productivity for solid/liquid sampling and imaging. One of the emerging trends in MS applications is the use of high-resolution MS in the quality control and characterization of biopharmaceutical therapeutics (2–5).
Agilent RapidFire 400 High-Throughput MS: The Agilent RapidFire 400 is an integrated autosampler for ultrafast sample cleanup based on automated SPE and introduction to MS. It can accommodate an integrated plate handler robot and optimized workflow for high‑throughput screening applications.
Agilent 6470B TQ-MS: This TQ-MS system is designed for reduced maintenance and high sensitivity. It has a mass range of 5–3000 amu, scan speed up to 17000 Da/s with a choice of five ion sources (electrospray ionization [ESI], jet stream technology [AJS], atmospheric‑pressure chemical ionization [APCI], multimode ionization [MMI], and NanoESI).
Agilent 6560 Ion Mobility LC/QTOF: The 6560 Ion Mobility QTOF LC–MS system delivers separation and selectivity by combining chromatography, ion mobility, and mass spectrometry. It can uncover structural changes of large proteins via collision-induced unfolding (CIU) and separation of in-class isomers via high-resolution demultiplexing (HRdm 2.0) and achieve ion mobility resolution over 200. Acquisition rates of up to 5 Hz allow for fast separation technologies such as UHPLC, SFC,and CE without compromising resolution.
Sciex ZenoTOF 7600 MS: This system has a Zenotrap that can perform electron activation dissociation with tunable fragmentation for biopharma and glycan analysis. It has a scan rate of up to 133 Hz with flow compatibility from 1 µL to 3 mL/min.
Shimadzu MALDI-8030: This is a compact benchtop dual-mode (positive and negative ionization) matrix-assisted laser desorption–ionization (MALDI)‑MS with a mass range up to 500,000, mass resolution >5000 full width at half maximum (FWHM), and mass accuracy 20–150 ppm. It is designed for imaging and life science applications.
Thermo Scientific TSQ Plus MS Portfolio: Thermo Scientific offers three TSQ Plus systems, including Fortis Plus, Quantis Plus, and Altis Plus. All TSQ Plus MS is offered with a new power supply delivering 5 ms polarity switching and an Active Reaction Collision Cell (ARC II) that maintains superior SRM acquisition speeds with improved low-mass product ions transmission. Specifications are: mass range (2–3000 Da, 2–2000 for Altis); scan speed (6000 SRM/s); resolution (0.4 Da, 0.2 Da for Altis); flow rate (100–1000 µL/min, 0.1–1000 µL/min for Altis). Interfaces available are ESI and APCI. TSQ MS is compatible with Chromeleon CDS and Xcalibur MS software.
Thermo Scientific Orbitrap Exploris MX Mass Detector: The high-resolution accurate mass detector is designed for operational simplicity in process development, quality control (QC), and production. Specifications are: mass range to 8000 m/z, MAM 2.0 Multi-Attribute method; flow range of 1–1000 µL/min; vent-free maintenance; 1–3 ppm mass accuracy; mass resolution of 180,000 at m/z 200; scan rate of 22 Hz, and a dynamic range >5000. It uses Chromeleon CDS for system control and data handling.
Thermo Scientific Orbitrap IQ-X Tribrid MS: Orbitrap IQ-X Tribrid MS is explicitly designed for small-molecule structural elucidation of metabolites and unknown compounds, offering researchers higher sample throughput with increased ease of use. This system benefits small molecule researchers in academia, pharmaceutical, and biopharmaceutical industries for applications including metabolomics and lipidomics research, leachable or extractable impurities identification, and forensic toxicology. Specifications are: dynamic range >5000; a mass range of 40 to 2000 m/z; mass accuracy 1–3 ppm; mass resolution up to 500,000 to 1 M FWHM.
Thermo Scientific Delta Q Isotope Ratio MS: This compact next-generation is a gas-phase isotope ratio MS (IRMS) that enables detailed analysis with greater precision and accuracy. This flexible system is designed for food, environmental, sports doping, and forensic testing applications. It supports a mass range up to 97 m/z.
Waters Acquity RDa Detector: The compact, compliance-ready TOF-MS analyzer with SmartMS technology is designed for routine accurate-mass measurements of small and large molecules by LC–MS. Dedicated workflows and compliance make this an attractive asset for late-stage development and quality control. Large- and small‑molecule workflows are available.
Current “Large Molecule Workflows” include intact mass analysis of proteins and oligonucleotides, subunit analysis of monoclonal antibodies (mAbs), peptide mapping/MAM (multi-attribute method), and released N-glycan analysis.
Current “Small Molecule Workflows” include accurate mass confirmation, impurity analysis, forced degradation studies, natural product screening, food integrity assessment, and seized drug screening.
Waters Xevo TQ Absolute: The XevoTQ Absolute is a tandem quadrupole MS that combines performance, efficiency, and confidence for quantifying challenging compounds in food, environmental, clinical, pharmaceutical, biomedical research, and chemical materials applications. It delivers up to fifteen times higher sensitivity for negatively ionizing compounds than its predecessor, and requires less power, bench space, and gas usage than other TQ MS.
Waters Select Series MRT: The Select Series MRT is a multi-reflecting TOF-MS that provides a unique combination of high resolution and specificity independent of scan speed, allowing scientists to identify compounds in complex samples confidently. Launched with a focus on imaging mass spectrometry, it supports both DESI and MALDI ionization, providing complementary information to enable scientists to explore molecular structures and functions through the precise identification and localization of individual molecules in a sample.
Chromatography Data System (CDS) and Other Software Products
The CDS marketing landscape has been described elsewhere (6).
ACD/Labs Method Selection Suite Version 2021: This Method Selection Suite has improved data imports from Shimadzu LabSolutions, Agilent OpenLab, and Thermo Chromeleon CDS. It has better data handling near gradient breakpoints when optimizing LC Simulator and provides more options in data display and improvements for peak integration and database searches.
Agilent OpenLab CDS v. 2.6: The updated features for this version include supports for cloud service, communication protocols changes, and updates on data integrity, control panel, sample scheduler, MS data integration, and instrument control drivers.
Chromperfect 8.2 CDS: The latest version of Chromperfect for Windows 10 has new features and performance improvements to analyze and report data from CDS, including Agilent OpenLab and ChemStation.
Clarity DataApex 8.4 CDS: This version of Clarity now has methods comprising of acquisition and processing portions, plus an improved audit trail and new control modules for Shimadzu gas chromatography (GC)–MS.
S-Matrix Fusion QbD Method Development Software: The latest version of the popular Fusion QbD software has added several vital enhancements, including a new Replication Strategy Experiment (aligned with USP<1220> and ICH Q14 draft); more flexible DoE experimental setup; full support for the new Waters column compartment; improved automated peak tracking and Method Operable Design Region (MODR) modelling/visualization; enhanced robustness simulation; improvements to analysis; and reporting to method validation.
Thermo Scientific Chromeleon CDS, Version 7.3.1: The latest release of Chromeleon CDS, version 7.3.1, is an IT-ready (with Cloud-First implementation), scalable enterprise solution. It is designed to be faster and more productive with reduced audit risks and enhanced compliance tools. The new version provides increased functionality for Thermo Scientific SQ, TQ, and high-resolution MS. It supports updated instrument control drivers for Thermo Scientific Vanquish Core and Neo, Waters HPLC, and Agilent/Shimadzu HPLC and GC.
Waters Empower Lab Management System: This informatics system combines Waters Empower Chromatography Data System (CDS) with Waters NuGenesis Lab Management System (LMS) software. The Empower LMS is a single-solution approach to data management. It provides a single repository for all lab data to help maintain regulatory compliance with data integrity. It tracks inventories of reagents, standards, and instruments, reduces time spent entering/reviewing data, and eliminates the need for a paper logbook.
Testing Kits
Waters SARS-CoV-2 LC-MS Kit RUO, Biomedical Research Standards and Kits: Waters LC–MS Research Use Only (RUO) kit can directly detect and quantify SARS-CoV-2 signature nucleocapsid (NCAP) peptides without amplifying the target analytes. The kit is designed to measure three proteolytic peptides derived from the virus’s NCAP protein. This method is optimized for Waters’ Acquity UPLC I-Class Plus and Xevo TQ-XS System and is fully compatible with the Andrew+ Pipetting Robot.
Summary and Commentaries
On 9 March 2022, I attended the virtual plenary lecture by Professor Albert Heck on an innovative approach to the ionization of biomolecules in their native states and its applications in profiling immunoglobulins of individual patient samples. It was one of the most exciting and relevant lectures I attended in recent years, delivered with simplicity and clarity. This kick-off lecture is part of a limited online technical programme for Pittcon 2022. Unfortunately, this year will be remembered as the first Pittsburgh Conference without an exposition in the history of the series. Nevertheless, instrumentation developments for separation science remain robust, as witnessed by a record of 40+ products introduced in one year amidst a pandemic. While most industrial sectors retracted, biopharmaceutical and bioscience research has grown significantly, fuelled by substantial demand for analytical instrumentation.
With the positivity rate for Covid-19 infections waning in most areas, we are seeing the light at the end of the tunnel and are yearning for an early return to a post-pandemic “normal”. I am hopeful for a live Pittcon 2023 in Philadelphia, where I can again roam for hours to witness a full array of new separation science products on the conference centre floors!
Acknowledgements
The author thanks the marketing staff of all manufacturers who provided timely responses to the LCGC questionnaires: Agilent (Andrea Otto), PerkinElmer (Kyle Oberdorfer), Shine (Kevin Pu), Shimadzu (Kevin MacLaughlin), S-Matrix (Richard Verseput), Thermo Fisher Scientific (Peter Zipfell, Scott Peterman, Daniel Hermanson, Oleksandr Boychenko), and Waters (Brian Murphy).
A special thanks to my reviewers for providing timely technical and editorial inputs to this article: Alice Krumenaker of T W Metals, Dave Bell of Restek, Andrea Otto of Agilent, Yongle Pang of GSK, Kevin MacLaughlin of Shimadzu, Michael Heidorn and Peter Zipfell of Thermo Fisher Scientific, He Meng of Sanofi, and Elizabeth Bergman, Isabelle Vu Trieu, Brian Murphy, and Lama Berro of Waters Corporation.
References
- D. Guillarme and M.W. Dong, Eds., Trends in Anal. Chem. 63(Special Issue), 1–188 (2014).
- M.W. Dong, HPLC and UHPLC for Practicing Scientists (Wiley and Sons, Hoboken, New Jersey, USA, 2nd Ed., 2019), Foreword and Chapter 4.
- M.W. Dong, LCGC North Am. 39(4), 172–178 (2021).
- M.W. Dong, LCGC Europe 33(4), 193–200 (2020).
- M.W. Dong, LCGC Europe 32(4), 196–203 (2019).
- R. Mazzarese et al., LCGC North Am. 37(12), 852–866 (2019).
About The Column Editor
Michael W. Dong is a principal of MWD Consulting.
Email: amatheson@mjhlifesciences.com

Determining the Effectiveness and Safety of Cinnamon Derivatives for Diabetes Treatment with HPLC
March 27th 2025Cinnamon and its byproducts have been used for many years because of their antidiabetic effect. In a joint study conducted by Gazi University (Ankara, Turkey) and Düzce University (Düzce, Turkey), high performance liquid chromatographic (HPLC) and thin-layer chromatography (TLC) analyses, macroscopic analyses, and enzyme inhibition assays on diabetes-related enzymes were performed on cinnamon samples to determine whether they are safe to use for health purposes.










