New Advice on an Old Topic: Buffers in Reversed-Phase HPLC
LCGC Europe
Buffers are commonly used in reversed-phase liquid chromatography (LC) to control the ionization state of analytes. However, the addition of buffers is much more complex than simple pH control. Complex equilibria exist between these mobile-phase additives, the analytes, the silica surface, and even the stationary phase in certain circumstances. The addition of mass spectrometry (MS) as a primary detection technique makes decisions about mobile-phase additives even more crucial. In this column instalment, we use a model set of analytes and selected applications to demonstrate the effects that buffers can have not only on the selectivity of a separation, but also on the sensitivity of a reversed-phase analysis when using MS detection.
Sharon Lupo and Ty Kahler, Restek, Bellefonte, Pennsylvania, USA
Buffers are commonly used in reversed-phase liquid chromatography (LC) to control the ionization state of analytes. However, the addition of buffers is much more complex than simple pH control. Complex equilibria exist between these mobile-phase additives, the analytes, the silica surface, and even the stationary phase in certain circumstances. The addition of mass spectrometry (MS) as a primary detection technique makes decisions about mobile-phase additives even more crucial. In this column instalment, we use a model set of analytes and selected applications to demonstrate the effects that buffers can have not only on the selectivity of a separation, but also on the sensitivity of a reversed-phase analysis when using MS detection.
The successful use of buffers can be critical for the retention and separation of ionic sample mixtures containing any combination of acidic, basic, or neutral compounds, by reversedâphase liquid chromatography (LC). According to the Brønsted-Lowry definition, an acid is a substance that donates a proton (hydrogen ion, H+) and a base is a substance that accepts a proton. The product that results when the acid loses the proton is called the conjugate base of the acid. The product that results when the base gains a proton is called the conjugate acid of the base.
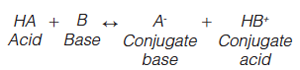
[1]
Acids differ in their proton-donating ability. Most acids are weak and only partially ionize in solution. The strength of an acid in solution can be expressed by the acid dissociation constant, Ka, as shown in equation 2. Acid strengths are typically expressed as pKa, or the negative logarithm of the acid dissociation constant. A stronger acid has a lower pKa (higher Ka) whereas a weaker acid has a higher pKa (lower K a) (1).
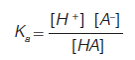
[2]
Buffer systems typically consist of a weak conjugate acid–base pair. For example, formic acid is a weak organic acid and ammonium formate is a salt containing its conjugate base. When in solution, this pair of compounds resists changes in pH because they contain both an acidic species to neutralize OH- ions and a basic one to neutralize H+ ions (2). In reversed-phase LC, additives such as formic acid, acetic acid, and ammonium hydroxide are commonly used to prepared mobileâphase solutions. Although they are not true buffers (at all operational pH values), these additives will maintain a relatively constant pH upon dilution.
Buffer Considerations
The Analyte: One of the primary functions of a buffer solution in reversed-phase LC is to maintain a constant mobile-phase pH so that acidic or basic analytes can be kept in a single ionization state. Using the Henderson-Hasselbalch equation, the relationship between pH and Ka is demonstrated:
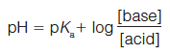
[3]
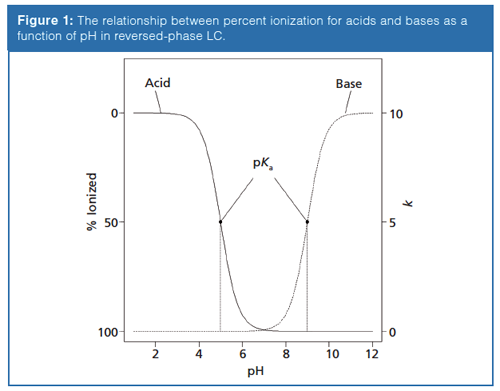
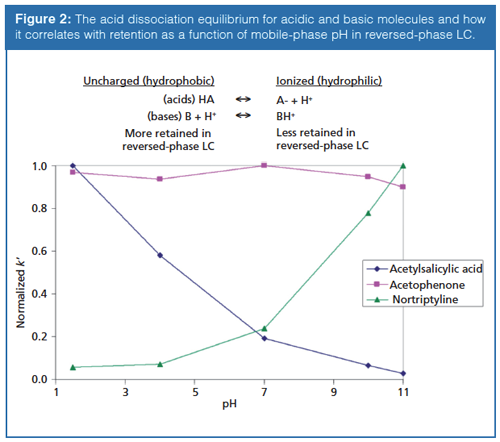
where [acid] and [base] refer to the equilibrium concentrations of the conjugate acid–base pair. As shown in Figure 1, the further the mobile-phase pH is from the pKa of a compound, the less impact small changes in mobile-phase pH will have on the compound’s ionization state. When the pH and pKa are equal, the compound is considered to be 50% ionized and small changes in mobile-phase pH will have more drastic effects on the compound’s degree of ionization. As a molecule increases in polarity, its retention will decrease. Therefore, when an acid or base becomes ionized the molecule becomes more polar, or hydrophilic, and retention is reduced (3). Differences in retention are demonstrated in Figure 2 where the retention factor (k′) of three representative compounds (acid, base, and neutral) are plotted against mobile-phase pH. In this example, acetylsalicylic acid (weak acid) loses a proton, or dissociates, and becomes ionized when the mobileâphase pH is increased. Alternatively, nortriptyline (weak base) gains a proton and becomes ionized as the mobile-phase pH decreases. The retention of acetophenone (neutral) remains largely unaffected by the changing mobile-phase pH. Through this example it becomes apparent that mobile-phase pH can be a powerful strategy for controlling selectivity in ionic sample mixtures. A common approach in reversed-phase LC is to adjust the mobile-phase pH so that ionizable compounds are in their neutral state for maximum retention. This means for acidic compounds, the mobile-phase pH should be below its pKa and vice versa for basic compounds. As a rule, the mobile-phase pH should be at least ±1.5 pH units above or below the pKa values of the analytes to avoid pH-related retention issues. Although using a mobile phase within 1.0 pH units of the compound pKa will provide chromatographers with the most control over selectivity, working in this region can result in an irreproducible separation since a minimal change in pH will result in a maximum change in retention.
When selecting a buffer for pH control, one must consider its buffer capacity and solubility. The ability of a buffer to maintain a constant pH is its buffer capacity, which is dependent on the buffer pKa value, buffer concentration, and the desired pH of the mobile phase. An appropriate buffer should have a pKa value within 1.0 unit of the desired mobile-phase pH. Outside of this range, buffering becomes less effective and an increased buffer concentration will be required to achieve the same buffer capacity (3). Likewise, buffer solubility must also be taken into consideration, particularly when gradient elution is performed; buffers can precipitate from solution when they are mixed with organic solvents. Buffer solubility is dependent on the buffer concentration, counter-ion, and organic solvent. Obviously, lower buffer concentrations will be more soluble. In general, ammonium salts are the most soluble and sodium salts are the least, while methanol tends to exhibit greater solubility than acetonitrile does.
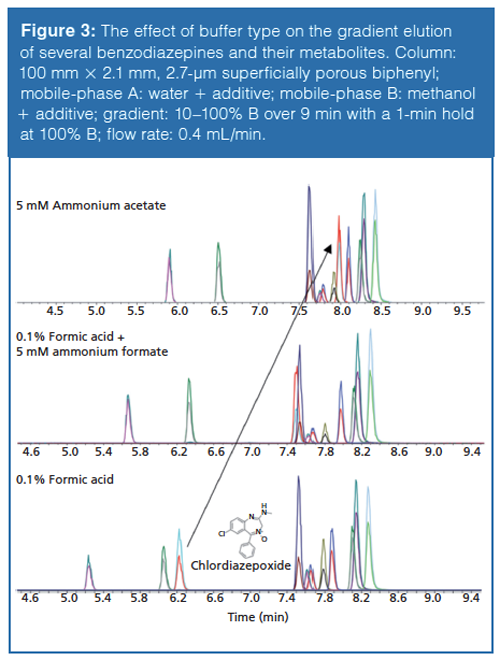
The impact of buffer type and the associated change in mobileâphase pH on relative retention is demonstrated in Figure 3. Here, a mixture of benzodiazepines and their metabolites are separated with gradient elution using three different mobile-phase modifiers of varying pH: 0.1% formic acid (pH ~2.7), 0.1% formic acid and 5 mM ammonium formate (pH ~3.0), and 5 mM ammonium acetate (pH ~6.8). As the pH of the mobile phase increases, several of the early eluting compounds (7-aminoclonazepam, 7-aminoflunitrazepam, and chlordiazepoxide) become more retained while the retention of the remaining benzodiazepines remains constant. In particular, chlordiazepoxide, with a pKa of ~4.6 (4), displays a drastic increase in retention that results in an elution order switch. The majority of the benzodiazepines are weak bases with pKa values ranging from 8 to 10. By raising the mobile-phase pH above its pKa, chlordiazepoxide becomes less ionized and more retained; orthogonal selectivity is observed as the mobile-phase pH crosses the pKa of chlordiazepoxide. The other benzodiazepines are unaffected because the mobileâphase pH does not approach their pKa and their ionization state remains unchanged. In this example, the use of different buffers allows the mobile-phase pH to be adjusted while maintaining sufficient buffer capacity by working within its buffer range.
The Column: In addition to analyte interactions, the effects of buffer on the silica support of the column must be considered. Most reversed-phase LC columns are stable for a mobile-phase pH between 2 and 8. Using mobile phases outside of this range can result in shortened column lifetime. Low-pH mobile phases (pH ≤2) can cause hydrolysis of the stationary phase from the silica support while high-pH mobile phases (pH ≥8) can dissolve the silica packing. Silica is less susceptible to dissolution when low concentration organic buffers are used in conjunction with low oven temperatures (5). Specialty columns exist that enable analysis outside this range by protecting the silica from chemical attack through steric hindrance or the use of hybrid silica supports.
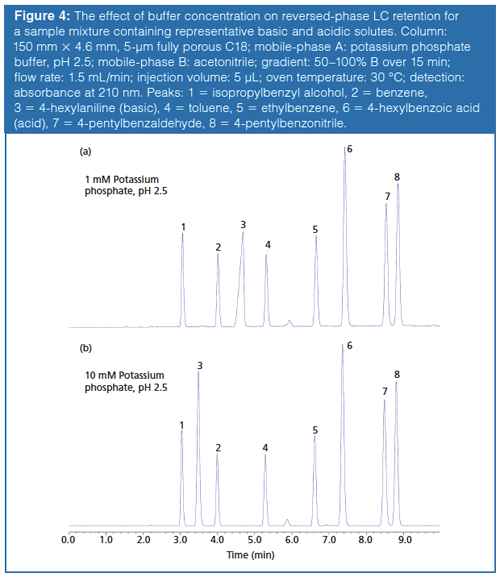
Buffer–silica interactions have been shown to reduce retention and improve peak shape of positively charged analytes by suppressing the ion-exchange retention mechanism that can occur between positively charged bases and the anionic silanols (pKa ~3.5–7) of the stationary phase support. This retention mechanism was pronounced in older type-A silica columns because of the presence of heavy metals that increased the acidity of the silanol groups. As a result, a higher concentration of ionized silanols occurred regardless of the mobile-phase pH causing severe peak tailing. Silanol ionization can be minimized by utilizing low-pH mobile phases; however, this can, in turn, protonate basic analytes. An increase in mobile-phase ionic strength has been shown to reduce silanol activity through masking of the silanol active sites. Ionic strength can be elevated through increased buffer concentration or by using a mobile-phase pH, which will optimize buffer capacity while maintaining the same buffer concentration. Most columns today consist of purer type-B silica, which is more reproducible with less tailing; however, they can still suffer from poor peak shapes. When protonated bases are analyzed in conjunction with low-pH mobile phases, charge repulsion can occur between the retained ionized molecules because of column overloading (6). This type of column overload can be overcome by reducing the injection volume or by increasing the mobile-phase ionic strength (7). In Figure 4, a sample mixture containing 4-hexylaniline (primary amine) is analyzed with two different phosphate buffer concentrations, 1 mM and 10 mM, both adjusted to pH 2.5. The mixture of 4-hexylaniline displays a peak shape indicative of column overload when 5 µL are injected with the 1 mM buffer concentration. When the same volume is injected with the 10 mM buffer concentration, the peak shape for 4-hexylaniline improves combined with a loss in retention. Here, peak shape is improved by increasing the ionic strength of the mobile phase. The increased buffer concentration results in a concurrent change in selectivity caused by a reduction in the available ionized silanols capable of ion exchange. Lacking a charge, the neutral compounds are unaffected by ionic forces of repulsion and attraction, and therefore their retention and peak shape remain consistent.
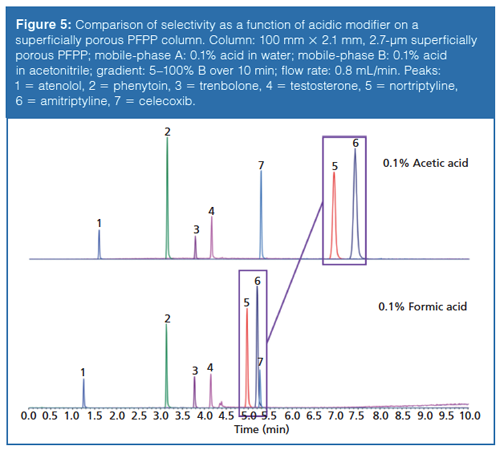
In some instances, buffers can enhance stationary-phase selectivity. This phenomenon is particularly true for perfluorinated phenyl phases where the electronegative fluorine ring can intensify the ion-exchange capability of the base silica over that of an alkyl column (8). Contrary to the previous example where increased mobile-phase ionic strength was used to suppress the ion-exchange retention of ionized bases, preferential selectivity of bases can be achieved by enhancing this mechanism. In a sample composed of acidic, basic, and neutral probes, reversed-phase selectivity is compared for water and acetonitrile mobile phases modified with 0.1% formic acid and 0.1% acetic acid on a superficially porous pentafluorophenylpropyl (PFPP) column (Figure 5). When switching from formic acid to acetic acid there is a dramatic increase in retention for nortriptyline (pKa ~10.5) and amitriptyline (pKa ~9.7), two tricyclic antidepressants both of which contain an amine functionality. The use of acetic acid as mobileâphase modifier results in a decrease in ionic strength and an increase in mobileâphase pH from ~2.7 (0.1% formic acid) to ~3.5. At this pH, it is likely that the number of active silanols will increase while nortriptyline and amitriptyline remain charged, resulting in an increase in retention. The neutral and acid probes are not capable of ion exchange with the silanols so their retention remains constant.
Mass Spectrometry Detection: Another consideration when choosing an appropriate buffer is the intended means of detection. Characteristics such as absorbance and volatility make some buffers incompatible with certain detectors. For example, if ultraviolet (UV) detection is performed, it is important to choose a buffer with a low UV cutoff to avoid a significant increase in the UV absorbance of the mobile phase (typically a buffer with a UV cutoff <210 nm is preferred). For liquid chromatography–mass spectrometry (LC–MS) methods, a volatile buffer is required to prevent excessive background noise, contamination, and fouling of the MS detector.
Sensitivity in LC–MS directly relates to ionization efficiency, or the effectiveness of producing gas-phase ions from analyte molecules in solution (10). There are several ionization techniques available; however, the focus of this discussion is on electrospray ionization (ESI). In ESI, positive and negative ions are separated by the presence of an electrostatic field at the tip of the sample capillary in the ionization source. In positive ESI, the negative ions are neutralized on the capillary wall, and positive ions drift downfield to the surface of the liquid front at the capillary tip where a Taylor cone is formed (11). At the surface of the Taylor cone, electrostatic repulsion overcomes the surface tension of the liquid and causes the cone to break up into small electrically charged droplets. These droplets travel towards the atmospheric pressure interface, and as they do, their surface area decreases because of evaporation of the solvent from the droplet. As a result, the surface charge density of the droplet increases. Once the droplet reaches a certain radius, called the Rayleigh limit (12), repulsion forces once again exceed the surface tension and cause an explosion of even smaller droplets to form (13). The process repeats itself until the droplet is so small that gas-phase ions are emitted (14).
ESI has many advantages, including its applicability to analytes over a large polarity range and its compatibility with large molecules or proteins and thermally labile compounds. The primary disadvantage of ESI is that it is susceptible to the reduction of detector response due to competition for ionization efficiency in the ionization source, also known as ion suppression (or enhancement in the case of elevated ion efficiency). There are multiple causes for this phenomenon, including competition for available charge or surface area of the droplet, increased surface tension, which can reduce the rate of solvent evaporation (15), coprecipitation of the analyte with nonvolatile solutes (16), and neutralization of the analyte by ion pairing with mobileâphase additives or by gas-phase reactions (17). Ionization by ESI is complex and can be influenced by the characteristics of the mobile-phase buffers selected. In many cases, the mobile-phase system that is optimal for analyte retention and resolution is not optimal for analyte ionization because neutral analytes tend to display the best retention in reversed-phase LC. Therefore careful selection of mobile-phase buffer type and concentration is paramount for a sensitive and selective assay.
Common choices for LC–MS mobile-phase additives are acetic acid, formic acid, and their ammonium salts. These buffers are volatile and allow for adequate coverage of the working pH range of 2–8 for reversed-phase LC. Alternatively, ammonium hydroxide or ammonium bicarbonate can be used for high pH applications (pH >8). If pH <2 is required, trifluoroacetic acid is a viable option; however, strong acids such as this are capable of ion-pairing, which can have deleterious effects on method sensitivity and can be difficult to flush from the instrument if used for prolonged periods of time.
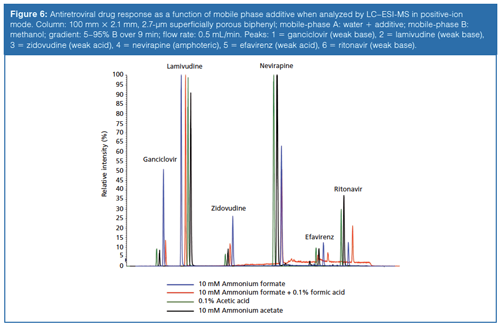
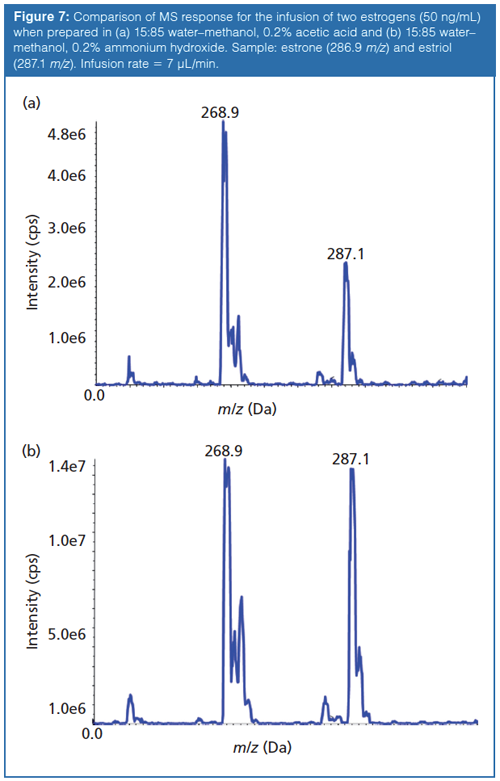
In addition to increasing the reproducibility of the chromatographic method, these additives can serve to enhance ionization. The effects of different buffers on ionization is not always well understood and can be compound dependent, as shown in Figure 6. The response for several antiretroviral drugs analyzed by LC–ESI-MS in positive-ion mode are compared as a function of additive composition in the mobile phase. Ganciclovir, a weak base, displays optimal response with a 10 mM ammonium formate buffer. Surprisingly, so does zidovudine, a weak acid. Lamivudine appears to ionize well regardless of the buffer used, whereas efavirenz shows poor ionization under all circumstances. The overall best responses are achieved with a 0.1% acetic acid modification. It would be reasonable to believe that an acidic modifier would provide the best analyte response in ESI positive-ion mode because of its ability to donate protons, while a basic modifier would be preferred in negative-ion mode as a proton acceptor. This theory holds true for the negative ionization of two neutral estrogens, estrone and estriol (Figure 7). The response for the estrogens triples when they are prepared in diluent containing 0.2% ammonium hydroxide compared to one that contains 0.2% acetic acid. However, this is not always the case. It has been shown that abundant protonated molecules can be produced in basic conditions and abundant deprotonated molecules in acidic conditions. This phenomenon is referred to as wrong-way-round ionization (18).
Buffer salts containing ammonia (for example, ammonium formate or ammonium acetate) can increase the ionization efficiency of polar neutral compounds that cannot be ionized on their own. Ammonium adducts can boost the ionization efficiency of these compounds and can be formed by including a constant supply in the mobile phase (1–2 mM). In a recent example from our laboratory, we analyzed digoxin and digitoxin at low levels. These two cardiac glycosides exhibit very poor sensitivity in the absence of ammonium buffers; however, they show significantly enhanced sensitivity through adduct formation. Often the formation of adducts is unwanted since they can diminish the signal of the protonated molecule [M+H]+. Sodium and potassium adducts are ubiquitous in LC–MS since contamination of metal ions can occur from glassware, solvents, and analysts themselves. One way to reduce the level of metal adducts in ESI positive-ion mode is to lower the pH of the mobile phase with formic acid. The excess in protons provided by the acid will drive the majority of ion formation to the protonated molecule [M+H]+ (19).
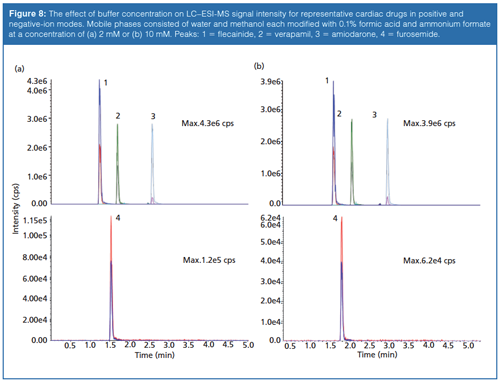
The concentration of mobile-phase modifiers used in LC typically range from 10–100 mM; however, loss in signal has been observed in ESI at this level. To avoid ion suppression, buffer concentrations not exceeding 10 mM are recommended for LC–MS applications. The inverse relationship between response and buffer concentration in ESI may be attributed to an increase in repulsive forces because of increased charge density. These forces cause spreading of the spray plume in the source and a reduction of ions in the centre of the spray with ultimately less ions collected by the atmospheric pressure ionization (API) source for detection (20). Decreased sensitivity could also be caused by competition at the surface of the droplet due to an increase in bulk ions from the mobile phase (21). The effects of buffer concentration are demonstrated in Figure 8 where cardiac drugs flecainide, verapamil, and amiodarone were analyzed by LC–ESI-MS in positive-ion mode and furosemide was analyzed in negative-ion mode. As the concentration of ammonium formate is increased in the mobile phase from 2 mM to 10 mM, the overall intensities in positive and negative-ion mode decrease approximately 9.3% and 48.3%, respectively. Mallet and colleagues (22) devised experiments to benchmark common volatile mobile-phase additives and their effect on ESI response. Although there was a strong compound dependency, for the mobile-phase acids, bases, and buffer salts tested, Mallet found that acidic additives and buffer salts showed an inverse relationship between concentration and ESI signal for acidic and basic compounds analyzed in positive and negativeâion mode; as the concentration is increased a decrease in response is observed. On the other hand, ammonium hydroxide displayed a positive correlation with an increase in sensitivity for basic compounds in positive-ion mode with increased concentration (22). In this scenario, ionization is believed to take place through proton transfer reactions in the gas-phase, since an increase in electrolytes has been shown to cause suppressed ionization of analytes while in the liquid-phase (20). The relationship reverses itself once again for the analysis of acids in negative-ion mode using ammonium hydroxide with a decrease in response observed with an increase in concentration of the additive.
Conclusion
It is clear that the relationship between analyte retention, ionization, and the mobile-phase buffer system is complex. For improved retention, the application of acid–base theory as it relates to the analyte structure, pKa, and analytical column chemistry is required. In reversedâphase LC, buffers can be used to neutralize charged acids and bases for
improved retention. In turn, an understanding of LC–MS ionization theory needs to be used to avoid unnecessary ion suppression. Although ionization efficiency is largely analyte specific, the use of mobileâphase additives in low concentrations can improve ESI response. Predicting the effects a particular additive will have on a reversed-phase LC method is not absolute; however, the correctly chosen buffer can help create a sensitive, selective, and reproducible method.
References
- J. McMurry, Organic Chemistry, 3rd Edition (Brooks/Cole Publishing Company, Pacific Grove, California, USA, 1992), pp. 50–53.
- T.L. Brown, H.E. LeMay, Jr., and B.E. Bursten, Chemistry The Central Science, 6th Edition (Prentice Hall, Englewood Cliffs, New Jersey, USA, 1994), pp. 629–630.
- L.R. Snyder, J.J. Kirkland, and J.W. Dolan, Introduction to Modern Liquid Chromatography, 3rd Edition (John Wiley & Sons, Hoboken, New Jersey, USA, 2010), pp. 304–331.
- T.H Shayesteh, M. Radmehr, F. Khajavi, and R. Mahjub, Eur. J. Pharm. Sci.69, 44–50 (2015).
- J. Nawrocki, J. Chromatogr. A779, 29–71 (1997).
- S.M.C Buckenmaier, D.V. McCalley, and M.R. Euerby, Anal. Chem.74, 46–72 (2002).
- D.V. McCalley, J. Chromatogr. A1075, 57 (2005).
- D.S. Bell and A.D. Jones, J. Chromatogr. A 1073, 99–109 (2005).
- C.R. Mallet, A. Lu, and J.R. Mazzeo, Rapid Commun. Mass Spectrom.18, 49–58 (2004).
- J.S. Page, R.T. Kelly, K. Tang, and R.D. Smith, J. Am. Soc. Mass Spectrom. 18, 1582–1590 (2007).
- G.I. Taylor, Proc. R. Soc. Lond. A.280, 383 (1964).
- L. Rayleigh, Philos. Mag. 14(1), 184 (1882).
- A. Gomez and K. Tang, Phys. Fluid.6, 404 (1994).
- P. Kebarle, J. Mass Spectrom.35, 804 (2000).
- I. Fu, E.J. Wolf, and B.K. Matuszewski, J. Pharm. Biomed. Anal.18, 347 (1998).
- R. King, R. Bonfiglio, C. FernandezâMetzler, C. Miller-Stein, and T. Olah, J. Am. Soc. Mass Spectrom.11, 942 (2000).
- M.H. Amad, N.B. Cech, G.S. Jackson, and C.G. Enke, J. Mass Spectrom. 35, 784 (2000).
- S. Zhou and K.D. Cook, J. Am. Soc. Mass Spectrom.11, 961 (2000).
- F. Klink, MS Solutions #3, Sepscience.com/Information/Archive/MS-Solutions.
- R. Kostiainen and T.J. Kauppila, J. Chromatogr. A 1216, 685–699 (2009).
- R. Kostiainen and A.P. Bruins, Rapid Commun. Mass Spectrom. 8, 549–558 (1994).
- C.R. Mallet, Z. Lu, and J.R. Mazzeo, Rapid Commun. Mass Spectrom.18, 49–58 (2004).
Sharon Lupo joined Restek in 2010 as an LC Applications Chemist. While in this position, she focused on developing LC–MS/MS applications and providing LC technical support for the environmental, food safety, and clinical markets. Currently, Sharon is a Senior Scientist for the LC Product Development group and uses her market knowledge and analytical skills to develop new and innovative LC products. Sharon has nearly 20 years of experience in the field of liquid chromatography. Prior to joining Restek, she was a principal investigator, study director, and analytical chemist for good laboratory practice (GLP) and good manufacturing practice (GMP) regulated environmental, bioanalytical, and pharmaceutical studies.
Ty Kahler joined Restek as a Senior Innovations Chemist in 2008 and has since become the Manager of the HPLC R&D and Applications group. In addition to overseeing the HPLC lab, his responsibilities include LC methods development, quality investigation and implementation, and phase design and research. Before joining Restek, Ty worked as a manager, study director, and principal investigator for a contract research laboratory conducting methods development, validation, and routine analysis of pharmaceuticals and agrochemicals. He has been in the field of liquid chromatography for more than 17 years, including time as a quality chemist and instrumentation metrologist.
David S. Bell is a manager in pharmaceutical and bioanalytical research at MilliporeSigma (formerly Sigma-Aldrich/Supelco). With a B.S. degree from SUNY Plattsburgh and a PhD in analytical chemistry from The Pennsylvania State University, Dave spent the first decade of his career within the pharmaceutical industry performing analytical method development using various forms of chromatography and electrophoresis. During the past 20 years, working directly in the chromatography industry, Dave has focused his efforts on the design, development, and application of stationary phases for use in HPLC and hyphenated techniques. In his current role at MilliporeSigma, Dr. Bell’s main focus has been to research, publish, and present on the topic of molecular interactions that contribute to retention and selectivity in an array of chromatographic processes. Direct correspondence to: dave.bell@sial.com

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












