Morphological Comparison of Silica-Based Monolithic and Particulate Beds by Confocal Laser Scanning Microscopy
LCGC North America
This analysis offers insight for optimizing the structure of monoliths.
Confocal laser scanning microscopy enables the nondestructive, three-dimensional (3D) imaging of silica-based columns. The key morphological features responsible for the kinetic column performance can then be identified directly from the physically reconstructed bed structure, independent of size and form of the underlying structural element. We demonstrate this by comparing the bed morphology of a silica monolith with that of a sub-2-µm packing in capillary column format (20 µm i.d.). Our analysis reveals the main structural problem of the silica monolith and shows the direction along which its optimization should progress to reach the kinetic efficiency of a sub-2-µm packing. The morphological monitoring enabled by this approach offers constructive guidance to any optimization efforts in the preparation of silica-based monolithic and particulate columns.
Porous silica in the form of particulate or monolithic beds is the most popular stationary phase support for small molecule separations (1). Monolithic columns have overcome the limitations of particulate beds with respect to permeability and mass transfer resistance, but often show disappointing separation efficiencies (2); presently, even the improved second generation of silica monoliths (3,4) cannot compete with well-packed particulate beds (5–7). A similar situation in which a theoretical concept has not yet brought the expected outcome in practice is the size reduction of the structural elements of a bed. Smaller particle and domain sizes do not automatically guarantee better columns, only higher back pressure (8). Shrinking the structural elements yields more efficient columns only when the homogeneity of the bed can be conserved in the process (9–11). The bed morphology, specifically the distribution of the interstitial void space where eddy dispersion takes place, largely determines the kinetic column performance (12–14). The heterogeneity of a monolithic or particulate bed contributes to eddy dispersion on different length scales (15–17): the size of the individual channels (flow-through pores) between two neighboring solid elements (transchannel contribution) (18,19), a size equivalent to 1–2 particle diameters or domains (short-range interchannel contribution) (18,19), and the column radius (transcolumn contribution) (4,7,20–23).
With particulate beds, the size of each eddy dispersion contribution can be determined from chromatographic data and linked to the respective morphological features (14,23,24). The method cannot be readily transferred to monolithic beds because the theory of eddy dispersion was first developed for discrete spheres as the underlying structural elements (15). This does not mean that the origins of eddy dispersion are fundamentally different in monolithic beds, only that the comparison of particulate and monolithic beds by their column efficiency and hydraulic permeability cannot get at the morphological roots of the observed chromatographic output (25–28). The same applies to comparative studies of silica-based monolithic columns with different domain sizes, for example, in efforts to reduce the macropore size (29–31). For particulate beds, the particle size is used as a normalization parameter when comparing the chromatographically determined efficiencies of individual columns. This normalization works because the size of the particles and that of the interstitial flow-through pores are in a fixed relationship, so that when the size of the particles is reduced, the size of the flow-through pores also decreases. But the corresponding elements of a monolithic bed, skeleton thickness and macropore size, can be altered independently (2); therefore, the domain size, although often treated as the analog of the particle size, is not a suitable normalization parameter for monolithic beds.
Yet, the morphology of particulate and monolithic beds can be analyzed through three-dimensional (3D) imaging methods: With local resolution, image analysis delivers an accurate characterization of the interstitial void space from individual pores up to the column cross-section. In our group, we have used confocal laser scanning microscopy (CLSM) for silica-based (hard) materials (4,7,32–35) and serial block-face scanning electron microscopy for polymer-based (soft) materials (36). Using CLSM, we have found the morphological cause behind the varying quality in a series of silica monoliths (34), investigated the influence of the capillary internal diameter on the packing morphology (7), and compared core–shell and fully porous particles with respect to the wall effects in packed capillaries (35). Excepting one study (4), our CLSM investigations so far have been conducted with capillary columns because this format enables direct access to the bed morphology; that is, it is not necessary to extrude the bed from its conduit and cut the extruded bed into smaller samples. Also, a smaller column internal diameter allows reconstruction of longer bed segments and the amount of CLSM data involved is still manageable.
In this article, we demonstrate how the morphologies of chromatographic beds can be directly and quantitatively compared by image analysis, independent of size and form of the underlying structural element of the bed. As examples we selected two capillary columns of identical internal diameter (20 µm): a silica monolith and a packing of sub-2-µm particles.
Experimental
The investigated fused-silica capillary columns (20 cm × 20 µm), a tetramethoxysilane (TMOS) monolith prepared as described in the literature (30), and a packing of C18-modified, 1.7 µm Acquity BEH particles (Waters Corporation) prepared as shown in the literature (7) were kindly provided by Takeshi Hara and Dr. Bernd M. Smarsly (monolith) of Justus-Liebig-Universität, Giessen, Germany, and James P. Grinias, Laura E. Blue, and Dr. James W. Jorgenson (packing) of the University of North Carolina at Chapel Hill. All elements of the experimental approach have been described in detail elsewhere (7,32–34), so only the salient points are repeated here. For image contrast, the particulate bed was stained with Bodipy 493/503 (Life Technologies), a dye that physisorbs to the C18 chains, whereas the bare-silica skeleton of the monolith was chemically linked to the dye V450 (32). A 65-µm-long segment from the medium section of each column was reconstructed from a set of consecutive optical slices (image stack, resolution: 30 × 30 × 120 nm, Figure 1) recorded on a TCS SP5 confocal microscopy system equipped with a HCX PL APO 63×/1.3 GLYC CORR CS (21°) glycerol immersion lens (Leica Microsystems). The refractive index mismatch between lens and sample was minimized through flushing and embedding the investigated column with (as well as immersing the lens in) a 70:19:11 (v/v/v) liquid mixture of glycerol–DMSO–water, whose refractive index mimics the optical dispersion of fused silica. The remaining small mismatch was eliminated by a judiciously chosen cover slip ("type 0," 110 nm thickness). Contrast, signal-to-noise ratio, and resolution in the acquired images were improved by image restoration. The gray-scale images were then converted to binary data (in which each pixel belongs to either solid or void space) through segmentation. Images of the monolithic column were binarized by high-pass filtering; images of the particulate column were binarized by detecting the center of a particle and fitting a sphere with the appropriate diameter around it.

Figure 1: Physical reconstruction of the bed morphology of (a) a silica monolith and (b) a packing of sub-2-µm particles by confocal laser scanning microscopy. Segments (65 µm long) of each capillary column (20 µm i.d.) were reconstructed from image stacks of consecutive optical slices acquired parallel to the column axis.
Results and Discussion
Figure 1 schematically shows how the bed morphologies of the two capillary columns were physically reconstructed by CLSM. Images were acquired from parallel planes along the column axis. The resulting image stack represents a set of consecutive optical slices through a column, from wall to wall. The reconstructed segments cover 65 µm of each column. With the reconstructed morphologies available, our analysis of the two bed structures progressed from the pore scale to the column scale.

Figure 2: Void space characterization by inscribed spheres. Colors indicate the diameters of the largest spheres that can be fitted into the interstitial void space of (a) the reconstructed monolithic and (b) particulate bed without intersecting the solid phase (gray). (c) The probability density distribution of the inscribed-sphere diameters (shown with the corresponding statistical parameters) can be interpreted as a lower limit of the pore size distribution.
Pore-Scale Properties
The interstitial void space in a particulate or monolithic bed is a network of flow-through pores whose size determines the amount of transchannel dispersion in the column. Unfortunately, the concept of linking an eddy dispersion term to the scale of individual flow-through pores is easier to understand than it is to obtain an accurate pore size distribution (PSD). The standard experimental method, mercury intrusion porosimetry (MIP), assumes a network of cylindrical pores; in reality, the flow-through pores in a column do not have a constant diameter, but often widen behind a constricted entrance (the pore neck). That MIP measures the diameter of the pore neck rather than the size of the actual pore is known as the ink-bottle effect (37). Considering this drawback, an MIP-determined PSD should be interpreted with caution. With the reconstructed morphology of a packing (Figure 1), an accurate image of the interstitial void space is available, but that still poses the question: How do you define a pore within an open pore network or how do you arrive at the PSD from the reconstructed morphology? One possibility is the inscription of spheres into the void space (38,39). Figure 2 shows the results of this approach applied to the reconstructed beds. Each void voxel was assigned the diameter of the largest sphere that could be inscribed at this location without intersecting with a solid voxel. Figures 2a and b show a monolithic and particulate bed, respectively, with the void space colored according to the diameters of the locally inscribed spheres. Figure 2c quantifies the results with the probability density distribution of the inscribed-sphere diameters for each voxel location in the void space. As expected, the inscribed-sphere diameters in the monolithic bed are, on average, more than twice the size of those in the particulate bed. The sub-2-µm packing shows a rather narrow and symmetrical distribution of inscribed-sphere diameters, whereas the monolith has a very broad, negatively skewed distribution. The latter reflects that fluctuations in skeleton thickness lead to an irregular solid–void interface whose description requires a larger number of smaller spheres. Although the inscribed-spheres approach has visual appeal and targets pores as opposed to pore necks like MIP, the results are inaccurate. Inscribing spheres into the void space probes the adaptation of the pore space to a fixed, idealized geometry more than the dimensions of the flow-through pores themselves, which makes the resulting PSD only a lower limit of the range in which the true PSD lies. An accurate, though less immediately comprehensible, method to describe the interstitial void space distribution in a porous medium was introduced by Courtois and colleagues (40) for chromatographic beds. Here, the particle-to-particle (or skeleton-to-skeleton) distances in the bed are measured by chords that are extended in two opposite directions from random positions in the interstitial void space (Figure 3a). Chords are generated and their lengths collected until the resulting histogram, known as a chord length distribution (CLD), remains constant. The CLDs of the reconstructed beds (Figure 3b) were established from 106 chords each and are statistically over-determined. The one-dimensional (1D) chords meticulously scan the complex geometry of the solid–void border in a porous medium, so that the CLD fully captures the morphology of the reconstructed interstitial void space. This accuracy is one essential advantage of the CLD method over the inscribed-spheres approach (and MIP). The other important advantage is that chords may occasionally reach into the next flow-through pore; such longer chords then contain information about the pore vicinity.

Figure 3: Quantitative void space characterization by chord length distributions. (a) From random points P1 to Pn in the interstitial void space of the reconstructed monolithic bed, the linear skeleton-to-skeleton distance (for the particulate bed, the particle-to-particle distance) is determined in 16 equiangular directions (green lines); chords that reach beyond the image boundary are rejected (red dashed lines). (b) k-gamma fit to a distribution of 106 chords and the corresponding statistical parameters that measure the pore-scale properties (average pore size, transchannel, and short-range interchannel heterogeneity) of the reconstructed monolithic and particulate bed.
The interpretation of the received CLDs in terms of eddy dispersion contributions is helped by the circumstance that the CLDs follow a k-gamma function. The k-gamma function (41) was delineated as a descriptor of the void space distribution in computer-generated, coagulated colloids of monosized spheres (42). With the first physical reconstructions completed, we discovered that the k-gamma function was also a good fit for the CLDs of the interstitial void space of experimental particulate and monolithic beds (4,33–35,43). The k-gamma function is defined by the mean and the standard deviation of the CLD:
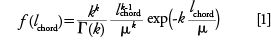
where lchord is the chord length, Γ is the gamma-function, µ denotes the statistical mean of the distribution, and k = (µ/σ)2 relates the mean µ to the standard deviation σ of the distribution. Alternatively, µ or the mode of the CLD (which is reasonably close to the mode of the inscribed-spheres determined PSD, Figure 2c) can be used to characterize the size of the flow-through pores. In the monolithic bed, the flow-though pores (µ = 3.60 µm, mode = 1.97 µm) are about twice the size than those in the particulate bed (µ = 1.78 µm, mode = 0.91 µm). The size of the transchannel contribution to eddy dispersion scales with the µ-value of the CLD (34). From the comparison of the received µ-values, we can predict that transchannel dispersion in the monolith is larger than in the sub-2-µm packing; in fact, it is comparable to the transchannel dispersion in a ~4 µm packing.
The value of k is dominated by the longer chords that make up the tail of the CLD and contain information on the local pore environment. Larger k -values represent a narrower distribution relative to µ; that is, a higher bed homogeneity on the length scale of 1–2 particle diameters or domains (4,33–35,43). The monolith (k = 2.22) is more homogeneous on the short-range interchannel scale than the sub-2-µm packing (k = 1.94), which would translate to a smaller contribution to eddy dispersion in the monolithic column. This finding agrees with our previous investigations: Among the capillary columns we have reconstructed by CLSM so far the particulate beds have fallen into the narrow range of k = 1.9–2.0 (33,35), whereas the silica monoliths have shown a wider range of k = 1.9–2.5 (34,43). The maximum observed value (k = 2.9) was obtained for an analytical TMOS monolith column of the second generation (4). Although our sample range is too small at present to predict the k -value range of monolithic beds, it is fairly reasonable to expect a narrow k -value range for well-packed particulate beds. These columns seem to possess a similar amount of order in the core region because the constraints of a dense packing near the random-close packing limit (44) leave little possibility for structural variation on the short-range interchannel length scale (18,35).
Column-Scale Properties
Radial heterogeneities in the interstitial void space distribution on the scale of the column diameter are a serious threat to the separation efficiency of any column, but are particularly critical in capillary chromatography. Porosity biases in a column translate directly into permeability and velocity biases (21,22). The extent to which such velocity extremes are experienced by an analyte and, thus, become apparent in the chromatographic output depends on the ratio of column internal diameter to column length (23). Analytical columns are too short for their internal diameter to complete equilibration of the analyte over the column cross-section; thus, when the analyte arrives at the column outlet, it has not felt all transcolumn velocity biases existing in the bed. Consequently, the efficiency of analytical columns suffers less from existing radial heterogeneities than that of capillary columns, whose kinetic performance discloses every heterogeneity in the packing (7,16,19,22,45).

Figure 4: (a) Characterization of transcolumn heterogeneity by radial porosity profiles. The bulk porosity (εbulk) of the reconstructed capillary segments was estimated from the interstitial porosity in the core region (r = 6â10 µm), in which the stationary phase material is randomly distributed. (b) Subtraction of εbulk from the porosity profiles eliminated the large difference in average interstitial porosity between the monolithic and the particulate bed. The scalar resulting from integration over the area covered by these curves is the integral porosity deviation (IPD, equation 2). The IPD quantifies the local porosity variation over the column cross-section relative to the bulk porosity.
We first calculated the external porosity, εext, of the reconstructed capillary segments from the amount of void voxels divided by the total amount of voxels. The results of εext = 0.69 for the monolith and of εext = 0.42 for the sub-2-µm packing are average values, such as could also be obtained by inverse size exclusion chromatography or Donnan exclusion (46), but say nothing about the void space heterogeneity. Figure 4a shows the radial porosity profiles ε(r), the local porosity ε as a function of the distance r from the column wall (r = 0), obtained for both beds. The radial porosity profile of the monolith is relatively flat compared with that of the sub-2-µm packing, which shows the typical "damped oscillations" profile of particulate beds (7,22,35). Because of the huge difference in external porosity, εext, the porosity profiles of the two beds cannot be directly compared. Instead, the local porosity variation with respect to the average porosity in the bulk (core) region of each bed is compared. The bulk region of each bed (r = 6–10 µm) is indicated in Figure 4a. In the monolith, bulk (εbulk= 0.70) and external porosity (εext = 0.69) are very close in value. In the sub-2-µm packing, the bulk porosity is εbulk = 0.36, while the local porosity at the column wall is ε = 1. To quantify the local porosity deviation with respect to the bulk porosity, we subtracted εbulk from the porosity profiles and integrated the remaining deviation over the column radius (Figure 4b). The resulting scalar is the integral porosity deviation (IPD) (7,35):
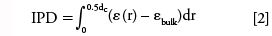
where r is the distance from the column wall and d c is the column internal diameter. The small IPD value of the monolithic bed (IPD = –0.08) reflects the flat porosity profile and the negative sign indicates a lower porosity at the column wall than in the bulk. The ~1-µm-thick low-porosity region originates from the wetting layer of monolithic material at the capillary wall and is followed first by a region of higher porosity and then by another low-porosity region (r = 2.2–4.6 µm). This often-observed morphological feature of capillary silica monoliths (34) is formed during the drying stage, when the bed shrinks and locally disconnects from the material layer at the wall. The high-porosity region reflects where the bed is stretched thin, and the low-porosity region reflects where the material has accumulated. Whereas the porosity profile of the monolith reflects its preparation history, the profile of the sub-2-µm packing and its IPD value (IPD = 0.28) reflect the fundamental limitations on the packing of hard spheres against a locally flat, hard column wall. For the first 3–5 µm from the capillary wall, the bed has a much higher porosity and also higher order than in the randomly packed bulk (7). The absolute IPD value of the monolith is 3.5 times lower than that of the sub-2-µm packing. The high radial homogeneity of the monolithic bed promises low transcolumn dispersion and constitutes a substantial structural advantage of the monolithic over the particulate format. Unfortunately, columns prepared in the past have often disappointed in this regard (2), which has led to reservations against silica monoliths in general.
Conclusions
We compared the bed morphologies of two 20 µm i.d. capillary columns with different silica-based support structures following their physical reconstruction by CLSM. According to our analysis, the monolith is more homogeneous than the sub-2-µm packing on the short-range interchannel and on the transcolumn scale, but loses significantly on the transchannel scale, with flow-through pores twice the size of those in the sub-2-µm packing. If the macropore size could be reduced while maintaining the present level of short-range interchannel and transcolumn homogeneity, the monolith would become superior to the sub-2-µm packing. The physical reconstruction of the bed morphology by CLSM allows users to monitor the morphological consequences of slurry packing and monolith preparation on scales of all lengths relevant to eddy dispersion: A CLD of the reconstructed interstitial void space delivers the parameters µ and k , which indicate the average pore size and the heterogeneity in the direct vicinity of a pore, respectively, while radial porosity profiles track changes in transcolumn heterogeneity. Decreasing µ while conserving k and the IPD value should be the goal of monolith preparation. Reduction of the transcolumn heterogeneity as indicated by a drop of the IPD value would have the strongest impact on the kinetic performance of slurry-packed particulate columns. Beyond all questions, image analysis provides pivotal insight into the morphological foundations of column performance. Besides guiding academic and industrial researchers in the preparation of better high performance liquid chromatography (HPLC) columns, the CLSM-based physical reconstruction of chromatographic beds delivers realistic models for flow and transport simulations to derive accurate mass transfer relationships for HPLC (19,47,48).
Acknowledgment
This work was supported by the Deutsche Forschungsgemeinschaft DFG (Bonn, Germany) under grants TA 268/5 and TA 268/6. The authors thank Takeshi Hara and Dr. Bernd M. Smarsly of Justus-Liebig-Universität, Giessen, Germany, and James P. Grinias, Laura E. Blue, and Dr. James W. Jorgenson of the University of North Carolina at Chapel Hill for generously providing the studied capillary columns.
References
(1) K.K. Unger, R. Skudas, and M.M. Schulte, J. Chromatogr. A 1184, 393–415 (2008).
(2) G. Guiochon, J. Chromatogr. A 1168, 101–168 (2007).
(3) K. Cabrera, LCGC North Am. 30(S4), 30–35 (2012).
(4) K. Hormann, T. Müllner, S. Bruns, A. Höltzel, and U. Tallarek, J. Chromatogr. A 1222, 46–58 (2012).
(5) J.R. Mazzeo, U.D. Neue, M. Kele, and R.S. Plumb, Anal. Chem. 77, 460A–467A (2005).
(6) F. Gritti and G. Guiochon, J. Chromatogr. A 1217, 1485–1495 (2010).
(7) S. Bruns, J.P. Grinias, L.E. Blue, J.W. Jorgenson, and U. Tallarek, Anal. Chem. 84, 4496–4503 (2012).
(8) J.J. Kirkland and J.J. DeStefano, J. Chromatogr. A 1126, 50–57 (2006).
(9) J.W. Jorgenson, Annu. Rev. Anal. Chem. 3, 129–150 (2010).
(10) L.E. Blue and J.W. Jorgenson, J. Chromatogr. A 1218, 7989–7995 (2011).
(11) J. Chamieh, Y. Zimmermann, A. Boos, and A. Hagège, J. Colloid Interface Sci. 340, 225–229 (2009).
(12) F. Gritti and G. Guiochon, J. Chromatogr. A 1218, 3476–3488 (2011).
(13) F. Gritti and G. Guiochon, J. Chromatogr. A 1252, 31–44 (2012).
(14) F. Gritti and G. Guiochon, LCGC North Am. 30(7), 586–595 (2012).
(15) J.C. Giddings, Dynamics of Chromatography, Part 1: Principles and Theory (Marcel Dekker, New York, New York, 1965).
(16) S. Khirevich, A. Höltzel, A. Seidel-Morgenstern, and U. Tallarek, Anal. Chem. 81, 7057–7066 (2009).
(17) F. Gritti and G. Guiochon, J. Chromatogr. A 1216, 4752–4767 (2009).
(18) S. Khirevich, A. Daneyko, A. Höltzel, A. Seidel-Morgenstern, and U. Tallarek, J. Chromatogr. A 1217, 4713–4722 (2010).
(19) D. Hlushkou, S. Bruns, A. Höltzel, and U. Tallarek, Anal. Chem. 82, 7150–7159 (2010).
(20) E. Vandre, R.S. Maier, D.M. Kroll, A. McCormick, and H.T. Davis, AIChE J. 54, 2024–2028 (2008).
(21) J.A. Abia, K.S. Mriziq, and G.A. Guiochon, J. Chromatogr. A 1216, 3185–3191 (2009).
(22) S. Khirevich, A. Höltzel, A. Seidel-Morgenstern, and U. Tallarek, J. Chromatogr. A 1262, 77–91 (2012).
(23) F. Gritti and G. Guiochon, J. Chromatogr. A 1262, 107–121 (2012).
(24) F. Gritti and G. Guiochon, J. Chromatogr. A 1217, 5137–5151 (2010).
(25) N. Tanaka, H. Kobayashi, N. Ishizuka, H. Minakuchi, K. Nakanishi, K. Hosoya, and T. Ikegami, J. Chromatogr. A 965, 35–49 (2002).
(26) U. Tallarek, F.C. Leinweber, and A. Seidel-Morgenstern, Chem. Eng. Technol. 25, 1177–1181 (2002).
(27) E. Oláh, S. Fekete, J. Fekete, and K. Ganzler, J. Chromatogr. A 1217, 3642–3653 (2010).
(28) F. Gritti, N. Tanaka, and G. Guiochon, J. Chromatogr. A 1236, 28–41 (2012).
(29) M. Motokawa, H. Kobayashi, N. Ishizuka, H. Minakuchi, K. Nakanishi, H. Jinnai, K. Hosoya, T. Ikegami, and N. Tanaka, J. Chromatogr. A 961, 53–63 (2002).
(30) T. Hara, H. Kobayashi, T. Ikegami, K. Nakanishi, and N. Tanaka, Anal. Chem. 78, 7632–7642 (2006).
(31) R. Skudas, B.A. Grimes, M. Thommes, and K.K. Unger, J. Chromatogr. A 1216, 2625–2636 (2009).
(32) S. Bruns, T. Müllner, M. Kollmann, J. Schachtner, A. Höltzel, and U. Tallarek, Anal. Chem. 82, 6569–6575 (2010).
(33) S. Bruns and U. Tallarek, J. Chromatogr. A 1218, 1849–1860 (2011).
(34) S. Bruns, T. Hara, B.M. Smarsly, and U. Tallarek, J. Chromatogr. A 1218, 5187–5194 (2011).
(35) S. Bruns, D. Stoeckel, B.M. Smarsly, and U. Tallarek, J. Chromatogr. A 1268, 53–63 (2012).
(36) T. Müllner, A. Zankel, C. Mayrhofer, H. Reingruber, A. Höltzel, Y. Lv, F. Svec, and U. Tallarek, Langmuir 49, 16733–16737 (2012).
(37) A.B. Abell, K.L. Willis, and D.A. Lange, J. Colloid Interface Sci. 211, 39–44 (1999).
(38) T. Hildebrand and P. Rüegsegger, J. Microsc. 185, 67–75 (1997).
(39) M.Y.M. Chiang, F.A. Landis, X. Wang, J.R. Smith, M.T. Cicerone, J. Dunkers, and Y. Luo, Tissue Eng. Part C: Meth. 15, 65–76 (2009).
(40) J. Courtois, M. Szumski, F. Georgsson, and K. Irgum, Anal. Chem. 79, 335–344 (2007).
(41) T. Aste and T. Di Matteo, Phys. Rev. E 77, 021309 (2008).
(42) I. Schenker, F.T. Filser, L.J. Gauckler, T. Aste, and H.J. Herrmann, Phys. Rev. E 80, 021302 (2009).
(43) D. Hlushkou, S. Bruns, A. Seidel-Morgenstern, and U. Tallarek, J. Sep. Sci. 34, 2026–2037 (2011).
(44) V. Baranau, D. Hlushkou, S. Khirevich, and U. Tallarek, Soft Matter 9, 3361–3372 (2013).
(45) A. Daneyko, S. Khirevich, A. Höltzel, A. Seidel-Morgenstern, and U. Tallarek, J. Chromatogr. A 1218, 8231–8248 (2011).
(46) S. Jung, S. Ehlert, M. Pattky, and U. Tallarek, J. Chromatogr. A 1217, 696–704 (2010).
(47) F. Gritti and G. Guiochon, J. Chromatogr. A 1221, 2–40 (2012).
(48) A. Daneyko, D. Hlushkou, S. Khirevich, and U. Tallarek, J. Chromatogr. A 1257, 98–115 (2012).
Stefan Bruns, Alexandra Höltzel, and Ulrich Tallarek are with the Department of Chemistry at Philipps-Universität Marburg, in Marburg, Germany. Direct correspondence to: tallarek@staff.uni-marburg.de

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.