Micropipette Tip-Based Sample Preparation for Bioanalysis
LCGC Europe
The micropipette tip containing solid phases is a relatively new sample preparation format that permits the handling of microlitre and submicrolitre amounts of liquid samples using the techniques of solid-phase extraction, dialysis and enzyme digestion. Phases are packed, embedded or coated on the walls of the pipette, permitting liquid samples to be moved and transferred without undue pressure drop or plugging. This column reviews the latest technologies in micropipette tip sample preparation used in the study of genomics, proteomics and metabolomics.
The move towards miniaturization in analytical chemistry has prompted the development of new formats for sample preparation. The micropipette tip format permits the handling of submicrolitre amounts of samples such as biological fluids. Solid-phase extraction (SPE) has been performed with various phases packed, embedded or coated on the walls of the pipette tip. The open flow architecture allows liquid samples to be moved and transferred without undue pressure drop or plugging. Many popular SPE techniques have been demonstrated including reversed-phase, ion exchange, hydrophobic interaction, hydrophilic interaction, immobilized metal affinity and affinity chromatography. Aside from SPE, micropipette tips have also been used to miniaturize dialysis and enzyme digestion. This month's instalment of "Sample Preparation Perspectives" reviews the latest technologies in micropipette tip sample preparation used in the study of genomics, proteomics and metabolomics.
In the past 10 years, the structural determination of proteins by the use of mass spectrometry (MS) has created new opportunities and challenges in analytical chemistry and biochemistry. The newly developed techniques provide the potential to map human proteins similar to the way a first blueprint of the human genome was announced a few years ago. Yet, the Human Proteome Project has more challenges than the Genome Project because of the complexity of protein structure and post-translational modifications, which often consist of dynamic changes. MS, by providing protein detection sensitivity at levels of 1 amol or lower, represents a breakthrough technology for analysing the structures of proteins in a single cell.
One of the primary challenges of proteomics is to determine the structures of biologically functional molecules from among a pool of millions of proteins, each with its own dynamic structure and unique post-translational modifications. While MS provides detection sensitivity, proteins still need to be purified before analysis. Some scientists assert that the purification of proteins before MS analysis is unnecessary because, in their view, a representative fragment of a protein can be identified by MS and then matched against a computer database of protein sequences. Yet, the current limitation of this method is that it might miss the true functions of proteins based upon their post-translational modifications. On the other hand, the purification of proteins before MS analysis can also result in the loss of, or changes to, the post-translational modifications of proteins and thus represents many of the same limitations. Another challenge to pre-MS protein purification is obtaining sufficient quantities of the protein of interest.1,2 For example, in human plasma, 10 proteins represent 90% of protein weight and an additional 200 proteins represent 9% of the remaining weight. The remaining millions of proteins account for less than 1% of the protein weight of plasma.
Background
The first set of analytical tools, developed decades ago, were based upon the isolation of several grams of proteins to determine their structure. Today, we use most of the same techniques: adsorption, partition, ion exchange and affinity chromatography,3 metal chelation, electrophoresis (1-D or 2-D)4 and SPE, but we have reduced our scale to micro levels for increased sensitivity. For example, when 2-D-electrophoresis was developed, one could obtain about 2000 spots from a sample. Today, the resolution is not much better; one still obtains about 2000 spots out of a set of millions of proteins. Each spot could contain hundreds of thousands of proteins, to be further analysed.
Two techniques for the MS analysis of proteins include electrospray ionization (ESI) and matrix-assisted laser desorption–ionization (MALDI),5–7 and ESI is used to ionize biomolecules in solution, while MALDI is used to sublimate and ionize a sample in a crystalline, dry matrix via a laser pulse. ESI is used to analyse more complex protein and peptide mixtures after they have undergone chromatographic separation and has become more powerful by the use of new tools such as Picotip (New Objective Inc., Woburn, Massachusetts, USA) and MudPIT (multidimensional protein identification technology).8,9 Further advancements use radioisotopes to get the isotope coded affinity tag (ICAT).10
Figure 1: Examples of micropipette tips: (a) Varianôs OMIX and (b) the Glygen NuTip. [Images courtesy of (a) Varian Instruments and (b) Glygen Corp.]
MALDI is commonly used for the analysis of proteins after protease digestion,8 such as tryptic digestion. However, for analysis by MALDI and atmospheric pressure MALDI (AP-MALDI), samples should be free of salts, buffer and detergent. The most common steps for MALDI sample preparation are protein separation by 2-D electrophoresis. The spots containing the proteins of interest are cut and digested with proteases. Tryptic digestion is performed in a buffer, which contains salts along with buffer and detergents from the gel. In some instances, 2-D electrophoresis poses limitations and the protein of interest is separated by HPLC or micro- or nano- LC and microfractions are collected. In both instances, the protein is digested with proteases in the presence of detergents, urea, buffers and salts. This initial separation process results in the presence of many other ions or molecules that reduce (suppress) the signal strength upon MALDI analysis.

Figure 2: Types of micropipette tips.
Micropipette Tip Technology
The first commercially available micropipette tip–based micro SPE (ZipTip) was developed by Millipore (Bedford, Massachusetts, USA) in the late 1990s. The original ZipTip contained C18 chromatographic media embedded in a polymer. The C18-based ZipTip was used successfully in the purification of peptide mixtures from buffers and salts. In recent years, several manufacturers have developed micropipette tip products (Figures 1 and 2) that can be used for the purification of small amounts of peptides using different chromatographic materials (Table 1).
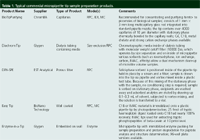
Table 1: Typical commercial micropipette tip sample preparation products (continued).
Micropipette tip–based SPE purification, concentration and selective isolation (affinity, metal chelate) of proteins and peptides is now an essential tool for MALDI and for other advanced analytical techniques.11 One of the main advantages of using micropipette tips is that they can be used with micropipettors or in liquid-handling automation. This has resulted in the routine use of micropipette tip–based SPE in bioanalytical labs, and it is easily adapted for use in high-throughput screening applications with commercially available xyz liquid-handling systems.
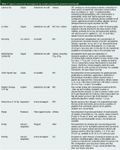
Table 1: Typical commercial micropipette tip sample preparation products.
Reversed-phase chromatography micropipette tips: The most commonly used hydrophobic (reversed-phase) chromatographic medium is octadecylsilane (C18) bonded to silica particles. Some manufacturers also offer C8, C4, C2 and phenyl media. Based upon their hydrophobicity, proteins in aqueous media are adsorbed on the chromatographic particles. After the adsorption step, the micropipette tips are washed with distilled water to remove excess salts and other molecules not adsorbed by the C18 phase. After washing, the adsorbed peptides are eluted with a water–organic solvent mixture (containing 50–70% organic solvent, such as acetonitrile, methanol, ethanol or other water-miscible organic solvents). The elution solution sometimes contains trifluoroacetic acid for better protein or peptide recovery.
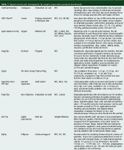
Table 1: Typical commercial micropipette tip sample preparation products (continued).
Hydrophobic chromatographic media based on silica are optimal for use in a pH range between 2 and 8 and are unstable beyond this range. Furthermore, most of the silica used in these tips has a 300 Ã pore diameter. POROS RP1 and RP2 media (Applied Biosystems, Foster City, California, USA), which are polymer-based materials with a larger pore size and wider range of pH resistance, thus might be better suited for the purification of certain protein and peptide samples. These polymer-based materials are available from different manufacturers. Monolithic-based micropipette tips include Omix from Varian (Palo Alto, California, USA) and MonoTip from GL-Science (Japan).
A disadvantage of hydrophobic micropipette tips is that they do not bind peptides or proteins in the presence of detergents. Therefore, a detergent-removal step is needed prior to the use of hydrophobic tips. Hydrophilic interaction chromatographic (HILIC) materials can be used to remove detergents from a sample in the same step in which peptides are purified (see HILIC).
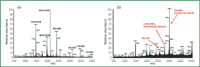
Figure 3: Glyco analysis by Hypercarb tip13: 500 fmol bovine fetuin purified by (a) NuTip-C18 and (b) mixed NuTip-C18âCarbon. (Images reproduced courtesy of Glygen Corp.)
Furthermore, some hydrophilic peptides such as phospho- (multiphosphorylated), glyco- and sulphatopeptides do not bind well to hydrophobic chromatographic materials. This represents a disadvantage of these media because more than 60% of human proteins are glycosylated or phosphorylated or a combination of both. Thus, peptides purified by hydrophobic interaction (reversed-phase chromatographic) media provide limited coverage. However, graphitic carbon can bind hydrophilic as well as hydrophobic molecules. Thermo Electron Corp. (Bellefonte, Pennsylvania, USA) offers Hypercarb, a graphite-based, commercially available chromatographic medium (Table 1) that can be used for the purification of some glycopeptides.12,13 A combination of C18 and Hypercarb (NuTip, Glygen Corp, Columbia, Maryland, USA) provides a wider range of glycopeptide coverage than the use of either C18 or Hypercarb alone (Figure 3). Hypercarb also can be used to purify phosphopeptides.14,15 The elution from the tip can be performed in a step gradient for partial separation (Figure 4).

Figure 4: Step gradient elution of bovine serum albumin peptides by using TrapôN Tip19: (a) full elution, (b) 10% acetonitrile, (c) 20% acetonitrile, (d) 40% acetonitrile and (e) 60% acetonitrile. (Images reproduced courtesy of New Objective Inc.)
Agilent Technologies (Palo Alto, California, USA) offers Cleanup C18 pipette tips as prescribed in the protocol for their Lys Tag 4H reagent.16 The tips with C18 silica embedded on the tip walls are used to remove excess Lys Tag 4H reagent before peptide analysis with MALDI-MS. The Lys Tag reagent enhances MALDI-MS signals for lysine-containing tryptically digested peptides, improving peptide mass fingerprinting results and making de novo sequencing of peptides more efficient. An example of a peptide with and without using the Lys Tag 4H reagent and protocol is shown in Figure 5, where the peptide was sequenced easily only after derivatization.
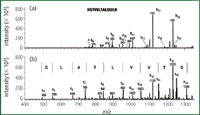
Figure 5: Use of Agilent Cleanup C18 Pipette Tip for removal of excess of Lys Tag 4H reagent: (a) no derivatization and (b) after derivatization with Lys Tag 4H reagent.16 (Courtesy of Agilent Technologies.)
Hydrophilic interaction chromatography micropipette tips: Available hydrophilic interaction chromatographic materials include diol, silica and PolyHYDROXYLETHYL A (PolyLC, Columbia, Maryland, USA). In hydrophilic interaction chromatography (HILIC), the sample is loaded on the material with 50–90% organic solvent in water, which results in the adsorption of proteins and peptides but not salts and detergents. The tips are then eluted with distilled water or a 10 mM concentration of a volatile acid such as formic acid. This process can be used for both proteins and peptides. The advantages of this method are that HILIC media do not bind urea or detergents and these impurities can be removed more easily compared with sample preparation based upon hydrophobic interaction chromatographic materials (Figure 6).
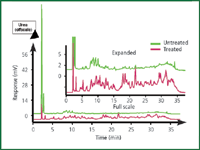
Figure 6: Removal of 1 M urea from a tryptic digest using HILIC-SPE with a TopTip micropipette tip followed by HPLC.18 Column: PolyHYDROXYETHYL A; mobile phase: TEAP buffer (pH 3); gradient: decreasing acetonitrile; sample: 200 μg transferrin tryptic digest.
Ion-exchange chromatography micropipette tips: Proteins and peptides are charged molecules because of the presence of acidic or basic amino acids and posttranslational modifications such as the addition of phosphorylated, sialylated, sulphated, amino and amino sugar functional groups. Weak cation exchangers are commonly used to purify the peptides containing a positive charge. Therefore, by changing the pH, certain peptides can be purified more easily than by using hydrophilic- or hydrophobic-interaction chromatography. For example, strong anion exchange micropipette tips can be used for the separation of sialyated and nonsialylated glycoproteins or peptides. A similar approach can be used for the purification of phospho- to nonphosphopeptides.
Immobilized metal affinity chromatography micropipette tips: Purification of phosphopeptides has created great interest in the immobilized metal affinity chromatography (IMAC) micropipette tip technique. Different metals such as Fe(III) and Ga(III) are incorporated in chelating media, which are then used for the phosphopeptides. In scientific meetings such as the American Society for Mass Spectrometry (ASMS), each year at least 100 posters are presented on "How to purify phosphopeptides?" There is no universal method for the purification of phosphopeptides.15,17 Ga(III) and Fe(III) form complexes with the phospho group of the peptide and the nonphosphopeptides do not bind to these affinity gels. After washing the micropipette tips, the peptides can be eluted using volatile buffers such as ammonium bicarbonate at pH 8–8.5. The advantage of using the volatile buffer is that peptides can be spotted directly onto the MALDI plate for MS. Other chromatographic materials such as TiO2 and ZrO2 also are used to bind the phosphopeptides for further purification. The phosphopeptides can also be purified by other methods such as the use of carbon- or anion-exchanger micropipette tips. The purification of phosphopeptides from nonphosphorylated peptides still poses a challenge and no single universal method is available. To study the difference between phosphorylated and nonphosphorylated proteins, it is very important to understand their metabolic pathways. By using Ni(II) IMAC, histidine-Tag proteins can be purified easily. A number of different chelating agents are used to form complexes with nickel. Different manufacturers have their own proprietary technology to bind His-Tag proteins. Most of the methods work reasonably well. However, in some instances, the leaching of metal has been reported and this trace metal can influence the analytical process.
Affinity chromatography micropipette tips: Recently, the affinity chromatographic method3 has been used to remove albumin from the plasma or serum. Albumin is the protein present at the highest concentration in plasma. Typical affinity ligands to remove albumin include dyes, antibodies or Protein A.
Another affinity chromatography method uses lectins for the specific purification or removal of glycopeptides or to separate glycosylated from nonglycosylated peptides. Different lectins are available for binding specific carbohydrates or glyco sequences. Therefore, by using a mixture of different lectins, many of the glycoproteins can be isolated or removed from a sample. Affinity chromatography is a promising method for removal of a particular group of proteins from a mixture of proteins, thus facilitating the identification of the spot in 2-D gels. The multiple affinity removal system (Agilent Technologies) removes the top six proteins from human or mouse serum.
Enzyme micropipette tips: A new method has been developed in which trypsin is adsorbed (noncovalently bound) on the surface of chromatographic media particles. The adsorption of trypsin can be accomplished on either a hydrophilic or a hydrophobic chromatographic medium. When adsorbed on hydrophilic media, trypsin can be eluted into the solution by using aqueous buffers. In case of trypsin adsorption on hydrophobic media, such as C18 chromatographic media, the trypsin activity takes place in the aqueous buffer while the trypsin remains adsorbed on the media and is not eluted. When C18 absorbed trypsin is used, peptides are further purified from the buffer using the hydrophobic properties of C18.
Combination of Dialysis and Chromatography
DiaChrom Tip (Glygen, Columbia, Maryland, USA) is a new design (Figure 7) in which chromatographic media is inside a dialysis tubing and the dialysis tubing is fitted in a micropipette tip (1–10 μL). The biological sample in solution can be purified in a few microlitres using two effects: one by the size exclusion capability of the dialysis tubing and the other by the affinity or adsorptive property of chromatographic materials placed inside the same micropipette tip. The desired sample, containing biomolecules such as DNA, proteins or other molecular components in solution, is brought in contact with the microdialysis capillary containing chromatographic material. The molecules smaller than the molecular weight cut off of the microdialysis capillary (10000 Da) will diffuse into the capillary and bind to the chromatographic material inside the capillary. The unbound material will be in equilibrium with the outside solution and can be washed away. By changing the properties of the outer solution, the molecules absorbed on the chromatographic material inside the dialysis capillary will be eluted and diffuse through the capillary wall. The sample will be cleaned from larger molecules as well as from smaller molecules that do not have the affinity to the chromatographic material. Furthermore, by using this technique, the sample is also concentrated.
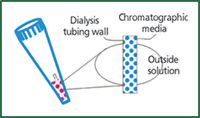
Figure 7: Diagram of a DiaChromTip, which combines dialysis and chromatography.
Micropipette Tip Spin Column
Harvard Apparatus (Holliston, Massachusetts, USA) offers a micropipette tip–based minispin column that contains a porous filter at the top and bottom of the chromatographic bed and can be used for larger sample volume as well higher binding capacity. These tip columns can be used to transfer liquid samples either by pressure, which can be applied by a pipetter, a syringe or vacuum. Glygen has introduced a filterless micropipette tip–based spin column. Instead of using the filter to hold the chromatographic material in the tip, the tip contains a slit at the bottom. This slit permits liquid to pass through but retains the chromatographic material (20–30 μm) in the tip. This eliminates the need for a filter and thus, there is no dead volume. By using these filterless tips of very small volumes (a few microlitres), minicolumns can be produced with any chromatographic media. Furthermore, the filter might have nonspecific binding to certain proteins, which can be a drawback when working with very small amount of proteins.
Multifunction Tips
In the future, multifunction tips might be needed to elucidate the structure of rare proteins that are present in even lower concentrations than presently handled. The use of different methods such as lectin or affinity tips in combination with enzyme tips and clean-up tips such as C18 will eliminate sample loss because of transport from one container to another by using different steps of purification.
Conclusion
Many different sample clean-up methods and varieties of micropipette tips have been developed. At present, the C18 tip is most commonly used for sample clean-up by desalting, detergent removal etc. In the future, these tips will be used for the study of metabolomics and functional proteomics such as protein–protein interaction, protein-DNA and RNA interaction. In addition, these tips can be used for the binding assays of small molecules as well as protein or peptide molecules for the development of new bioactive molecules. The advantage of micropipette tip–based sample preparation is that it can be adapted easily from single- or multichannel pipettes to liquid-handling robotics.
References
1. D.A. Wells and D.A. Weil, LCGC Eur., 16(11), 2–9, (2003).
2. D.A. Wells and D.A. Weil, LCGC, 21(6), 42–54, (2003).
3. W.C. Lee, and H.H. Lee, Anal. Biochem., 324, 1–10 (2004).
4. T. Wehr, LCGC, 19(7), 702–711 (2001).
5. J.B. Fenn et al., Science, 246, 64–71 (1989).
6. M. Karas, Anal. Chem., 60, 2299–2301 (1988).
7. R. Thomas, Spectroscopy, 16(1), 28–37, (2001).
8. R. Aebersold and M. Mann, Nature, 422, 198–207 (2003).
9. D. Lin, A.J. Alpert, and J.R. Yates, Am. Genom./Proteom. Technol., 1(1), 38–46 (2001).
(10) P. Gygi et al., Nature Biotechnol., 17, 994–999 (1999).
(11) R.E. Majors, LCGC, 23(4), 358–369 (2005).
(12) E. Stephens et al., Anal Chem., 76(8), 2343–2354 (2004).
13. A.K. Shukla, Am. Biotech. Lab., 21(11), 36–38 (2003).
14. M.R. Larsen et al., Mol. Cell. Proteom., 3, 456–465 (2004).
15. N.I. Taranenko et al. Am. Lab., 34–38 (May 2004).
16. W. Barrett et al., Agilent Application Note 5989-1089EN (2004).
17. D.T. McLachlin and B.T. Chait, Current Opinion Chem. Biol., 5(1), 591–1602 (2001).
18. A.J. Alpert and A. K. Shukla, ABRF poster 55 (2004).
19. C.J. Toher et al., ASMS poster 127 (2005).
Ashok Shukla is the president of Glygen Corporation, Columbia, Maryland, USA. He can be contacted at ashok@glygen.com "Sample Preparation Perspectives" editor Ronald E. Majors is business development manager, Consumables and Accessories Business Unit, Agilent Technologies, Wilmington, Delaware, USA and is a member of the editorial advisory board of LCGC Europe. Direct correspondence about this column to LCGC Europe, Advanstar House, Park West, Sealand Road, Chester CH1 4RN, UK, e-mail: dhills@advanstar.com

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.









