Microflow LC–MS-MS: The Past, the Present, and the Path Forward
LCGC North America
Microflow LC–MS-MS has seen a surge of attention, development, and popularity among research scientists and bioanalysts over the last few years. The potential of this technology to provide better sensitivity, less solvent waste, near-zero dead volume, and high through-put are a big part of this renewed interest. However, microflow LC techniques are hardly a new idea. More than 40 years ago, in 1974, a group at Nagoya University in Japan first developed a microcolumn liquid chromatography system, elements of which can be found in today’s commercial products. With the advances in technology over the last several years, development and implementation of this technique have been kicked into high gear. In this article, we discuss the history of microflow LC–MS-MS, the current state of the art, and where the future might lead for this rapidly growing technology.
Microflow liquid chromatography–tandem mass spectrometry (LC–MS-MS) has seen a surge of attention, development, and popularity among research scientists and bioanalysts over the last few years. The potential of this technology to provide better sensitivity, less solvent waste, near-zero dead volume, and high throughput are a big part of this renewed interest. However, microflow LC techniques are hardly a new idea. In 1974, a group at Nagoya University in Japan first developed a microcolumn LC system, elements of which can be found in today’s commercial products. In this installment of “Column Watch,” we discuss the history of microflow LC–MS-MS, the current state of the art, and where the future might lead for this rapidly growing technology.
During the last 20–30 years modern society has pushed technology to its limits. Computers that used to take up entire rooms now fit in the palm of your hand. This societal trend has been no different in the field of bioanalysis. New advances are continually being made and put into practice. Liquid chromatography coupled with mass spectrometry through electrospray ionization (LC–ESI-MS) is a relatively recent technological advance that has seen proliferation throughout many research and commercial applications, with widespread application in bioanalysis for the measurement of drugs, xenobiotics, and biomarkers from biological matrices. Since its inception, there has been an interest in making LC faster and more robust, minimizing waste and sample volume, and increasing sensitivity and selectivity. Microflow LC–MS (µLC–MS) has the potential to address and improve each of these areas.
There has been a recent surge of interest in the development of µLC–MS as hardware and technological capabilities have improved to meet the demands of ever-smaller sample volumes and the limited supply of precious materials. We are now at the point in which microscale LC–MS is not only possible, but feasible to implement on a much wider scale. Several vendors have recently poured resources into developing various types of “column-on-a-chip” microfluidics LC–MS platforms. Many of these are “plug-and-play” add-on components, designed to interface with currently established MS systems. Recent research has explored the use of such technology to quantify drug targets and assess the advantages of using µLC–MS as compared to the more commonly used high performance liquid chromatography (HPLC)–MS or ultrahigh-pressure liquid chromatography (UHPLC)–MS.
Interest in µLC–MS development and implementation is hardly new. In the mid-to-late 1970s we saw the first application of microflow LC from several groups. In this article, we discuss some of the history of microflow LC, its coupling with MS, the current work being done in this field, and future applications.
The Past
Interest and development of microflow chromatographic separations was first explored in the late 20th century. Ishii and colleagues (1–5) published a series of studies introducing their development of “micro high performance liquid chromatography” in the mid-1970s with use of slurry-packed PTFE microcolumns. This series of papers introduced an approach that used a precolumn to concentrate the analytes of interest before separation on a micro-HPLC column. Further work from Novotny, Scott, and Yang (6–10) provided key insights to the field of µLC. Scott’s work explored the use of microbore columns with high mobile-phase velocities. His group was able to separate a seven-component mixture in less than 30 s using a 0.2-µL injection volume and mobile-phase velocities of 12 cm/s, which, at the time, compared well with the capabilities of packed gas chromatography (GC) columns (7). Yang’s work explored the use of solvent modifiers to improve resolution, peak detection, and column selectivity when narrow-bore microparticle columns were put into use (10). The work of Novotny and his group explored the use of packed microcapillaries for LC separation and characterization of metabolic profiles. In these early uses of microflow LC, ultraviolet (UV) detectors were most commonly used. Also, as illustrated in Figure 1, there were three common types of columns typically used:
· Open microtubular columns, with thick outer walls and an internal diameter of 60 µm or less, drawn out to the desired length by procedures that were also typically used for GC glass columns. Stationary phase was bonded to the inner walls.
· Long microbore capillary packed columns, anywhere from ~45 cm to over 1 m in length with internal diameters of less than 1 mm.
· Packed microcapillaries (a hybrid of the microbore and the open microtubular columns) made from glass tubes that were first filled with the appropriate packing material and then drawn down to the desired diameter (8).
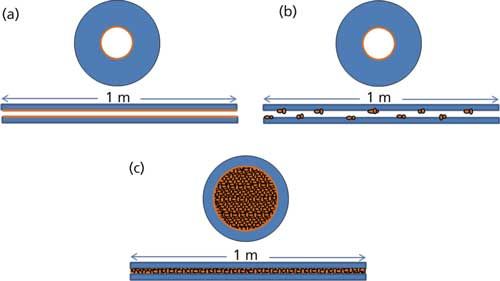
Figure 1: Illustrations of the different types of microcapillary columns in use during the late 1970s to late 1990s: (a) open microtubular column; (b) long microbore packed capillary column; and (c) a packed microcapillary column.
The first efforts were made to interface microcolumn LC with MS in the 1980s, using continuous-flow fast atom bombardment (CF-FAB) and magnetic-sector MS (6). One of the earliest breakthrough examples of this work came from Mosely and colleagues (11) in 1989 with their introduction of a system that interfaced both open tubular and packed microcapillary LC with a magnetic-sector MS detector. This type of interface was subsequently modified in later publications and became a useful desorption ionization technique in the analysis of polar and ionic compounds (12). In 1995, Li and colleagues (13) used a related approach with capillary LC and continuous flow secondary ion MS for discovery stage analysis of metabolites. ESI was also introduced early on as an interface technique for microflow columns to MS. Many of the µLC–ESI-MS applications were originally in the study of macromolecular structures, such as proteins. One such study, from Straub and colleagues (14), coupled perfusive-particle LC with ESI and quadrupole MS for the trace detection of β-lactam antibiotics residues in bovine milk. Other ionization interfaces included matrix-assisted laser desorption–ionization (MALDI) coupled with capillary gel electrophoresis for the analysis and identification of proteins, in combination with capillary LC–ESI-MS with protein digestion (15).
A common thread among all of these applications was the need to obtain increased sensitivity, reduce waste, or improve the lower limit of quantification (LLOQ). All advantages that µLC–MS can provide, along with the ability to analyze precious samples of limited quantity, reduce source contamination, reduce matrix effects, and produce a higher ESI response (16–19).
Recently, in 2009 and 2011, the Barran group at the Manchester Institute of Biotechnology combined nano-ESI with traveling wave ion mobility mass spectrometry (TW-IM-MS) in various protein studies (20,21), including the investigation of divalent cation binding to calmodulin (20). They also used this approach to obtain structural information to interrogate the preservation of the helical form of melittin from honey, in the gas phase, using the collision cross-section information obtained from IM, and the molecular mass information gathered from MS (21). Previous work from the Hill group (22) demonstrated the coupling of microbore LC with atmospheric pressure ESI ion mobility (µLC–ESI-MS) in which the lower flow rates resulting from microbore LC were shown to reduce matrix effects such as ionization suppression.
The Present
Today, research in the development of microflow LC–MS applications continues at a steady rate, although the primary focus in the bioanalytical field has been the development of UHPLC–MS-MS for robust analysis of samples in clinical and preclinical assays. With this technology firmly entrenched in bioanalytical contract research organization (CRO) applications, microflow LC has seen limited application, primarily in trial analysis to establish feasibility of use.
In recent years, however, there has been renewed interest and development in microflow LC technologies that are now gaining more traction in the bioanalytical community. These technological developments are largely the result of the availability of better hardware, the ability to micromanufacture the tiny components necessary, and improved software interfaces. Now, instead of 1-m column lengths, vendors have produced column-on-a-chip devices. These devices are often self-contained, and fully integrated with the source of an MS detector.
Some of the most recent work has explored the use of these more integrated microflow and nano-ESI separation technologies for quantitative analysis in pharmacokinetics (PK) studies. Wang and Bennett (23)recently compared the use of microflow LC coupled to high-resolution MS (µLC–HRMS) to more-conventional UHPLC–MS-MS (23). Their results suggest that µLC–HRMS does provide the expected benefits of lower detection limits and decreased matrix effects, such as ionization suppression. Other observed benefits included improved precision for more-reliable quantitation and decreased solvent waste because of ultralow flow rates. These benefits were achieved without significant extra effort for sample preparation and chromatographic separation. This study also illustrated one key disadvantage of µLC–MS-increased carryover because of low flow rates-representing a technological hurdle for future developments to overcome. This carryover was found to be both in the autosampler and the column. The authors suggest that, for this particular assay, it is likely that predilution of samples would be necessary, and that adequate rinsing between samples may become more important with the adoption of this technology into broader applications, dependent upon the sample and the system in use (23).
Two of the most recent publications from Zhang and colleagues (24) and Kleinnijenhuis and colleagues (25) investigated the use of chip-based µLC–ESI-MS-MS for PK analysis of monoclonal antibodies (mAbs). The former of these two studies also explored the use of microsampling of dried blood spots (DBS) with automated sample preparation to enable assay miniaturization and improve assay throughput (24). This study also used serial sampling, enabled by the low microsampling volumes required, in which multiple serial samples are collected from individual animals. This approach provides what the authors refer to as the benefit of the three “R’s” (reduce, refine, and replace). Fewer mice are required, and consequently less drug overall, and the resulting data are less variable because they eliminated some of the inter-individual differences that can arise when using large populations of subjects. In their approach, the authors used microsampling, microscale automation, and µLC–MS. Their results demonstrated how this approach can provide a significant savings in cost per sample (4x lower), solvent used per plate (10-fold decrease), and relative amount of injected sample required (>60x lower). In addition, they were able to achieve accuracy and precision within acceptable range for PK applications. Another encouraging observation was that very little modification of sample preparation procedures was needed in going from UHPLC–MS to µLC–MS analysis for this assay (24).
The work from Kleinnijenhuis’s group (25) explored the application and integration of µLC–ESI-MS-MS chip-based technology for the analysis of infliximab. They compared the use of this approach to their previously developed UHPLC–MS-MS approach for the infliximab assay (26). In this particular comparison, other potential drawbacks to µLC–MS-MS versus UHPLC–MS-MS were illustrated. Firstly, the investigators found it necessary to significantly improve the preprocessing of samples to optimize the µLC–MS-MS results and decrease interference from reagent compounds that were typically used in sample processing. Additionally, a significantly longer run time (16.8 min) was required to obtain optimal results with the µLC–MS-MS approach compared to the optimized UHPLC–MS-MS approach (4–8 min). A lengthy run time, however, can often be reduced in µLC–MS-MS with careful attention to optimal minimum dead volumes within the system. One key advantage that was demonstrated was the improvement in sensitivity that the µLC–MS-MS method provided (75 ng/mL versus 100–250 ng/mL in UHPLC).
In a unique application of microflow LC–MS-MS for small-molecule analysis, Christianson and colleagues (27) demonstrated the successful validation of a method for the analysis of methotrexate (MTX) within a regulated bioanalysis environment. In their analysis, an analytical method using a 50 mm x 0.5 mm, 3-µm dp microflow column was validated side by side with virtually the same method using a 50 mm x 2.1 mm, 3-µm dp HPLC column. The results demonstrated significant improvement in signal-to-noise ratio (S/N) using µLC–MS-MS compared to HPLC–MS-MS. Additional improvements illustrated in this work included the decrease in solvent volume used, a decrease in source contamination over time with a large number of injections, and the reproducibility of results after more than 400 injections. Overall, this work demonstrated a simple approach to development of rugged, accurate, and precise methodologies using µLC–MS-MS and simple conversion from HPLC to µLC–MS-MS (27).
Further work, from Christianson and Johnson and colleagues (27,28), validated the use of an in-source µLC column, again for µLC–MS-MS analysis of MTX in a regulated bioanalytical setting. The placement of the column within the source, as illustrated in Figure 2, provided several key advantages, such as significant reduction in dead volumes and a reduction of run times to under 5 min per sample. This study demonstrated an optimal run time, accuracy and precision, and robustness at a flow rate of 12 µL/min. As shown in their initial study, this new approach illustrated the relatively simple transfer of method from HPLC–MS-MS to µLC–MS-MS, with the key advantage of minimizing extra volume, integration into an already existing column source, and an alternative to column-on-a-chip approaches (28). The progression from HPLC–MS-MS to µLC–MS-MS to this fully integrated µLC–MS-MS is illustrated by the chromatographic spectral traces shown in Figure 3 (28).
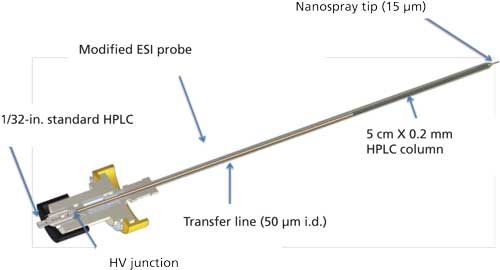
Figure 2: Illustration of a source-integrated µLC column (31).
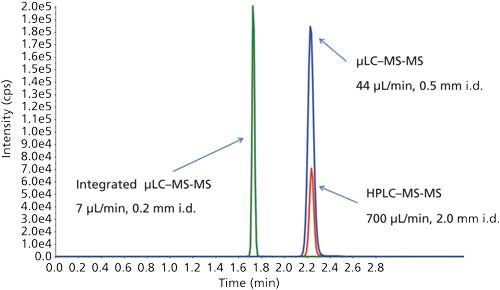
Figure 3: Comparison of HPLC–MS-MS (red trace), external µLC–MS-MS (blue trace), and integrated µLC–MS-MS (green trace).
The Path Forward
Modern technology advancements push scientific and physical boundaries, demanding the accomplishment of more with less and driving the microscaling of hardware, electronics, and other components. As this rapid advancement of technology continues, new approaches are emerging in the development of molecular-based medicine. Therapeutic, diagnostic, and treatment approaches are continually changing and adapting to the technological advancements of the modern era. As pharmaceutical companies shift their focus to development of biological therapeutics the demands of chromatographic analysis are ever increasing. The industry shift to green chemistry demands the decreased consumption of materials used in bioanalytical assays (29). The analysis of increasingly complex samples and increasingly scarce analytes will require increased sensitivity, improved resolving power, and better microsampling capabilities (24,26,29). Microflow LC–MS-MS technologies, using sub-50-µL/min flow rates and nano-ESI, are now poised to leverage these capabilities to bridge the gap from HPLC to µLC and from ESI to nano-ESI. Of course, more work is needed to establish the robustness of µLC–MS-MS and overcome the presently perceived disadvantages of long run times, increased carry-over, and complicated preprocessing of samples. The need to establish the ability of µLC–MS-MS to perform under the rigorous demands of a bioanalytical CRO laboratory, with nearly continuous sample throughput and continually changing solvent compositions from one assay to the next, is of critical importance (30). However, as more efforts are made to show crossover from HPLC and UHPLC to µLC for validated methods, bioanalytical CROs are more and more likely to adopt the technology into their everyday workflow. As illustrated in Figure 4, the path forward will depend on addressing these points with consistency, learning from past work that has been done, and applying our present knowledge to propel µLC–MS-MS into the future.
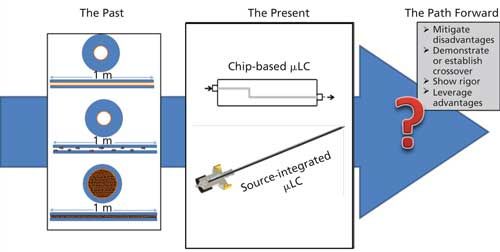
Figure 4: Past, present, and future perspectives for microflow LC–MS-MS analysis.
References
- D. Ishii, K. Asai, K. Hibi, T. Jonokuchi, and M. Nagaya, J. Chromatogr. A144(2), 157–168 (1977).
- D. Ishii, K. Hibi, K. Asai, and T. Jonokuchi, J. Chromatogr. A151(2), 147–154 (1978).
- D. Ishii, K. Hibi, K. Asai, and M. Nagaya, J. Chromatogr. A152(2), 341–348 (1978).
- D. Ishii, K. Hibi, K. Asai, M. Nagaya, K. Mochizuki, and Y. Mochida, J. Chromatogr. A156(1), 173–180 (1978).
- D. Ishii, A. Hirose, K. Hibi, and Y. Iwasaki, J. Chromatogr. A157, 43–50 (1978).
- J.P.C. Vissers, H.A. Claessens, and C.A. Cramers, J. Chromatogr A779(1–2), 1–28 (1997).
- R.P.W. Scott, P. Kucera, and M. Munroe, J. Chromatogr. A186, 475–487 (1979).
- M. Novotny, Clin. Chem.26(10), 1474–1479 (1980).
- T. Tsuda and M. Novotny, Anal. Chem.50(2), 271–275 (1978).
- F.J. Yang, J. Chromatogr. A236(2), 265–277 (1982).
- M.A. Moseley et al., Anal. Chem. 61(14), 1577–1584 (1989).
- M.A. Moseley, L.J. Deterding, K.B. Tomer, and J.W. Jorgenson, Anal. Chem.63(14), 1467–1473 (1991).
- C. Li, N. Chaurut, Y. Ducharme, L.A. Trimble, D.A. Nicoll-Griffith, and J.A. Yergey, Anal. Chem. 67(17), 2931–2936 (1995).
- R. Straub, M. Linder, and R.D. Voyksner, Anal. Chem. 66(21), 3651–3658 (1994).
- W.J. Henzel, T.M. Billeci, J.T. Stults, S.C. Wong, C. Grimley, and C. Watanabe, Proc. Natl. Acad. Sci. USA. 90(11), 5011–5015 (1993).
- S. Armenta, S. Garrigues, and M. de la Guardia, TrAC Trends Anal. Chem. 27(6), 497-511 (2008).
- R. Juraschek, T. Dülcks, and M. Karas, J. Am. Soc. Mass Spectrom. 10(4), 300–308 (1999).
- G. Valaskovic and N. Kelleher, Curr. Top. Med. Chem. 2(1), 1–12 (2002).
- E.T. Gangl, M. Annan, N. Spooner, and P. Vouros, Anal. Chem.73(23), 5635–5644 (2001).
- S.L. Shirran and P.E. Barran, J. Am. Soc. Mass Spectrom. 20(6), 1159–1171 (2009).
- H.V. Florance, A.P. Stopford, J.M. Kalapothakis, B.J. McCullough, A. Bretherick, and P.E. Barran, The Analyst (17), 3446 (2011).

Perspectives in Hydrophobic Interaction Temperature- Responsive Liquid Chromatography (TRLC)
TRLC can obtain separations similar to those of reversed-phase LC while using only water as the mobile phase.






