How Does It Work? Part III: Autosamplers
LCGC North America
A reliable autosampler is one key requirement for unattended operation of a liquid chromatograph.
A reliable autosampler is one key requirement for unattended operation of a liquid chromatograph.
This is the third installment in a series that examines how the different components of a liquid chromatography (LC) system work. We started with a look at pumps (1) and then considered mobile-phase mixing and degassing (2). This month we examine injectors and autosamplers.
When liquid chromatography advanced from open columns, which relied on gravity to drive the mobile phase through the column, to small-particle, packed columns operating at higher pressures, techniques for adding sample to a pressurized system were needed. Initially, modified septum-type injectors were tried, but these were not reliable and not compatible with even moderate pressures. Loop-type sample injection valves solved this problem. Initially these were used for manual injection, but nearly every LC system today relies on an autosampler to inject samples in an unattended manner.
Injection Valves
The heart of today’s autosampler is the injection valve. A schematic illustrating the key parts of the rotary injection valve is shown in Figure 1. The most common design is a six-port valve. The valve comprises a rotor, stator, loop, and various connections to the rest of the LC system. The rotor typically is made of a plastic disk with three kidney-shaped grooves cut into the surface; this design is represented by the curved passages inside the circle at the center of the valve in Figure 1. The rotor is connected to a handle or motor drive that allows it to be rotated through a 60° arc between a load and inject position. Pressed tightly against the rotor is a stationary disk called the stator. Typically, the stator is made of stainless steel or ceramic and is held tightly enough to the rotor that the seal is leak free up to the pressure limit of the LC system (for example, 400 bar or 6000 psi for a conventional LC system). The stator has six holes in it at 60° intervals that line up with the ends of the grooves in the rotor on one side of the stator and are attached to external components on the other side. The sample loop typically is made of stainless steel tubing with a length and internal diameter selected to hold a specific volume, such as 20 µL. The loop is attached to the stator with high-pressure fittings. The remaining fittings on the valve in Figure 1 are connected to the pump (p), column (c), sample inlet (s), and waste (w).
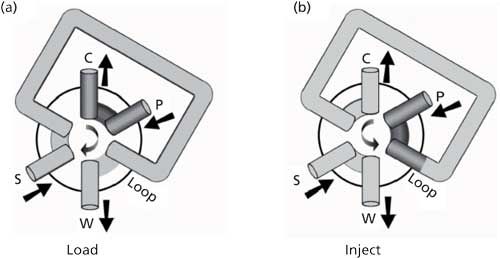
Figure 1: Six-port sample injection valve: (a) load position; (b) inject position.
In operation, the injection valve is used in a filled-loop mode or in a partially filled mode. In the filled-loop mode, the rotor is placed in the load position, as shown in Figure 1a. In this configuration, the pump output is connected directly to the column through one of the rotor passages; the sample loop connects the sample inlet to waste. Sample is introduced manually from a syringe or in an autosampler from an automated syringe. In the filled-loop mode, the sample volume exceeds the loop volume so that the loop is completely filled with sample and the excess flows to waste. So if a 20-µL loop is fitted to the injector, >20 µL is introduced to the loop. The valve is then rotated 60° (clockwise in Figure 1) to the inject position (Figure 1b). Now the loop connects the pump and column, so the mobile phase flowing from the pump sweeps the sample onto the column. Meanwhile, the sample inlet is connected to waste and can be flushed with cleaning solvent, if desired. For manual injections, the filled loop mode is very popular, because the injected volume is limited to the volume of the loop. That is, if 25 µL or 100 µL of sample is pushed into the injector, only 20 µL is injected. (There are some exceptions to this, but these are of little concern when autosamplers are used, so it is not discussed here. See earlier “LC Troubleshooting” discussions, such as reference 3, for more information.)
Because the loop volume dictates the injection volume in the filled-loop mode, this configuration is restricted to a single-size injection, and can be inconvenient when autosamplers are used. For this reason, autosamplers typically operate in the partial-fill mode. The valve configuration for the partial-fill mode is identical to that shown in Figure 1, except the loop size is selected to be larger than the desired injection volume. Perhaps the most common loop size is 100 µL. If we desire the same injection volume as in the previous example, 20 µL, we would push 20 µL of sample into the loop during the filling process. The remaining 80 µL of the loop would be filled with its prior contents, usually mobile phase or wash solvent. The rotor is then turned to the inject position and the contents of the loop are swept onto the column. Note that the flow direction through the loop is reversed between the fill and inject positions. This means that the last portion of sample placed in the loop is the first to reach the column. This is critical when small volumes (for example, 1–10 µL) are injected using a large loop (for example, 100 µL). If the fill and inject flow directions were the same, a small sample volume would flow through a large loop volume before reaching the column; it is likely that the sample band would spread enough to degrade the band width on the column.
In the partial-fill mode, the precision and accuracy of the injection process is controlled by the introduction of sample into the loop; in the filled-loop mode, the loop volume controls precision and accuracy. This difference usually means that for manual injection, better precision and accuracy are obtained by changing the sample loop to the desired volume rather than trying to control the amount of sample introduced. In theory, the same principle would apply to autosamplers, but the precision of the sample introduction system in autosamplers is such that partial- and filled-loop injection give similar precision.
Autosamplers
An autosampler is merely an automated injection valve with a mechanism to hold multiple samples and to load these samples into the sample loop as dictated by the system controller. Two popular configurations of autosamplers are in use today, the push-to-fill autosampler (Figure 2) and the needle-in-loop style (Figure 3). Other designs have been used in the past, but have not survived the evolution of LC equipment design.
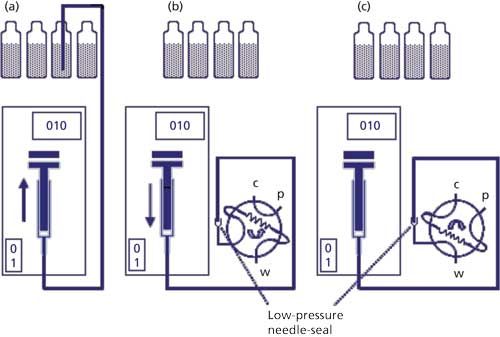
Figure 2: Push-to-fill autosampler design: (a) withdrawing sample from sample vial; (b) needle in low-pressure seal filling sample loop; (c) valve rotated for injection of sample.
Push-to-Fill
The push-to-fill autosampler is illustrated in Figure 2. Samples are placed in vials or 96-well plates and placed in a sample tray. A stepper-motor mechanism operates a metering syringe connected to a syringe needle via connecting tubing. Either the needle is moved to the vial location, usually via an x-y-z indexing mechanism, or the vial is moved to the needle. The needle pierces a sealing septum on the vial and sample is withdrawn through the needle into the holding tubing (Figure 2a). Next, the needle is withdrawn and inserted in a low-pressure sealing mechanism connected to the injection valve (Figure 2b). Sample is then dispensed into the sample port on the injection valve to either partially or fully fill the sample loop in the load position. The injection valve is then rotated to inject the sample into the mobile-phase stream (Figure 2c). After injection, the needle and connecting tubing usually is washed with a needle-wash solvent before the next injection.
The push-to-fill autosampler acts like an automated version of manual injection. If a small-volume sample loop is fitted on the injection valve, the autosampler can operate in the filled-loop mode. More commonly a large-volume loop (for example, 100 µL) is mounted on the valve and the partial-fill mode is used. Because the sample needle is separate from the connecting tubing, it is a relatively inexpensive part, and is easily replaced if it becomes blocked or bent. For maximum precision, some designs of push-to-fill autosamplers use syringes of different volume for small or large injection volumes; other designs use a single syringe size for all injection volumes. Because the needle and connecting tubing are not flushed with mobile phase and there is a short passage from the low-pressure seal to the injection loop, there will be some uninjected sample that is wasted with this autosampler design.
Needle-in-Loop
The needle-in-loop autosampler is illustrated in Figure 3. Most of the parts are similar to the push-to-fill design. The main difference is that the injection needle and connecting tubing are a single piece and also act as the sample loop. Sample is drawn from the sample vial into the needle–loop (Figure 3a), then the needle is inserted into a high-pressure seal and the injection valve is rotated to inject the sample onto the column (Figure 3b). Because the needle and loop are the same tubing, 100% of the sample that is drawn into the needle is injected. The needle-in-loop autosampler is especially useful for very small sample injections and when the amount of sample available is limited. If the needle becomes blocked or otherwise damaged, the entire needle–loop piece must be replaced, so maintenance costs can be higher with the needle-in-loop autosampler.
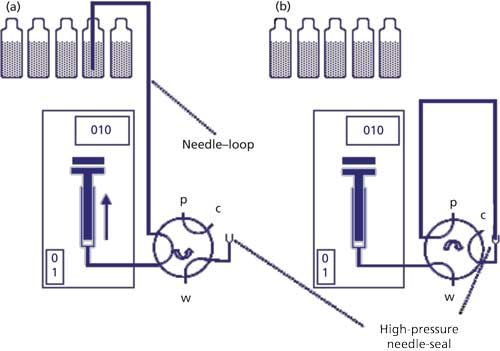
Figure 3: Needle-in-loop autosampler design: (a) withdrawing sample into the needle–loop; (b) needle in high-pressure seal with valve rotated to injection position.
Precision and Accuracy
In the days when manual injections were common and in the early days of autosampler use, injection precision and accuracy were poor enough that they affected overall method precision and accuracy. Today’s autosamplers operate with a high degree of precision because of the high-performance syringe metering mechanism. Most autosamplers operate routinely with relative standard deviations of <0.5% with injections of 5–10 µL or more. Imprecision of this magnitude seldom is a significant factor in overall method performance. It is a good idea to make several (for example, n = 6) injections of a well-behaved sample under controlled conditions to measure autosampler precision under ideal conditions. This performance test should be made at least annually to check injection precision and establish a baseline for future reference if precision problems are encountered. A drop in injection precision may be linked to a partially blocked needle, air bubbles in the tubing between the syringe and needle, a worn or poorly mounted syringe, leaks, or mechanical wear of autosampler parts. Careful elimination of the suspected problem sources one at a time will usually allow you to quickly isolate and repair or replace the problem component.
Autosampler injection accuracy is of less concern than is precision for most applications. This is because most methods call for injecting the same volume of sample for every injection-reference standards, quality control samples, and samples to be analyzed. If this is the mode of operation, it doesn’t matter if the accuracy is off a little, as long as the precision is acceptable. That is, if I want to inject 20 µL, but the autosampler only injects 18 µL, it usually doesn’t matter, because every injection will be 18 µL and the volume errors cancel. In a similar manner, autosampler linearity is of little concern if the same volume is used for every injection in a method. If accuracy and linearity are of concern, you can check accuracy by using a calibrated injection loop to compare response with filled-loop injection versus partial-fill injection. A series of different volumes of the same sample can be injected and the linearity of the response can be checked. However, as mentioned earlier, autosampler accuracy and linearity are seldom of much concern because it is rare to encounter an LC method that uses anything but constant-volume injections.
Problems
Today’s autosamplers are quite reliable, but they need care to remain that way. The most common problems are blockage, leakage, mechanical wear, and carryover.
Blockage
Sample vials typically use a septum-type closure to prevent sample from spilling or evaporating. Sometimes the needle can “core” a bit of the septum when the needle penetrates it. Thus, a small piece of septum can get lodged in the needle, blocking or partially blocking it. This may inhibit the mechanism of transferring sample from the vial to the LC system. Sometimes the septum fragment will be washed out of the needle during the next injection cycle; at other times it may be difficult or impossible to remove. If cleaning procedures don’t remove the debris, the needle may require replacement. If needle blockage is common, it may be possible to correct by using another septum material. My favorite septa are those with a silicone disk sandwiched between two thin pieces of PTFE. If these cause problems, a PTFE septum may be used, but these typically do not seal after the first injection is made, so they may not be acceptable with volatile samples or sample solvents when multiple injections are required. Many different septa materials and sealing designs are available, so it may take a little trial and error to find the best one for your application. If you need more ideas about what other users find best, consult one of the on-line discussion groups, such as Chromatography Forum (www.chromforum.org).
Leaks
There tend to be many tubing connections within the autosampler, and each of these may be susceptible to leakage, especially if system overpressure is encountered. Identifying the location of a leak usually is a simple matter of visual inspection, sometimes aided by poking the tip of a rolled-up laboratory tissue on suspect connections to see if a bit of liquid is present. Finger-tightened fittings are common, and these require special care when a leak is observed. When a leak results from an over-pressure condition, it is best to shut off the pump, then loosen each finger-tightened fitting, push the tubing into the fitting to ensure it is fully seated, then retighten the fitting. Sometimes when attempting to tighten a finger-tightened fitting with the system under pressure, the tubing can slip back a little, exacerbating the leak, or worse, leaving a small unswept passage within the fitting that will cause band spreading or carryover.
Wear
Any place in the LC system where friction between parts occurs, wear will take place and potentially cause leaks or other problems. Several parts of the autosampler are subject to such wear. The syringe used to meter sample will eventually wear and become less precise, less accurate, or leak. Replacement of the syringe is the simplest fix when this occurs.
The seal between the needle and injector valve for both the push-to-fill and needle-in-loop autosamplers will eventually leak. The push-to-fill seal usually is configured as a slip-fit seal that can be adjusted by tightening it when the needle is in the inject position; if this does not correct a leak, replace the seal. The needle-in-loop needle seal must withstand the full pressure of the LC system, so if it leaks, usually it must be replaced. Check the service manual for the autosampler for the part numbers and replacement techniques for either of these seals.
The injection valve rotor seal is made of a polymer-based material that will gradually wear out. With a conventional LC system (≤400 bar or ≤6000 psi), I have heard manufacturers quote 50,000 to 100,000 valve cycles before replacement is necessary. My personal experience confirms that this is reasonable, because I have rarely encountered worn valve rotors with today’s autosamplers. After all, 50,000 cycles represents 25,000 injections, which is more than some laboratories make in several years. Ultrahigh-pressure LC (UHPLC) systems operate at >400 bar (>6000 psi), and to maintain a leak-free seal between the rotor and stator, the two pieces must be pushed more tightly together than with lower-pressure systems. Thus, the lifetime of the rotor is expected to be less; I’ve heard quotes of 20,000-cycle lifetimes for such valves. In any case, failure because of rotor wear is infrequent enough that it is unlikely that replacement needs to be included in routine preventive maintenance plans. Instead, make sure the system software is counting the valve cycles (or estimate them from laboratory sample load), and when the cycle count approaches the numbers I mentioned above, keep your eye out for valve leaks that indicate a repair is needed.
Carryover
Carryover is the result of unintended injection of sample, such as when a solvent blank is injected. The source of such carryover typically is sample that is trapped in unswept areas of the flow stream, adsorbed on internal surfaces, or left in poorly washed autosampler parts. Nearly every autosampler has a wash mechanism-use it. Select a solvent that will readily dissolve the sample, so that any residual sample from one injection can be rinsed from the system before the next injection is made. In some designs of equipment, the wash solvent is injected and in others it is not. If the solvent is injected, it is important to choose a solvent that will not disrupt the chromatographic separation process. Sometimes the pH of the sample solvent can be selected to help enhance dissolution of residual sample.
If the wash mechanism is working properly and carryover persists, there may be a small, poorly swept cavity in the system. These can originate within tube fittings when an overpressure has occurred or the fitting is poorly assembled. Turn off the pump, loosen each fitting, and carefully reseat the tubing before tightening the fitting.
Conclusions
The autosampler is one of the more complicated components of the LC system. With many moving parts and often an x-y-z indexing system, there are many possibilities for mechanical failure. However, cleanliness and routine maintenance (consult the operator’s manual for specific recommendations) will make most autosamplers relatively trouble-free.
References
- J.W. Dolan, LCGC North Am. 34(5), 324–329 (2016).
- J.W. Dolan, LCGC North Am. 34(6), 400–407 (2016).
- J.W. Dolan, LCGC North Am.3(12), 1050–1052 (1985).

John W. Dolan
“LC Troubleshooting” Editor John Dolan has been writing “LC Troubleshooting” for LCGC for more than 30 years. One of the industry’s most respected professionals, John is currently the Vice President of and a principal instructor for LC Resources in Lafayette, California. He is also a member of LCGC’s editorial advisory board. Direct correspondence about this column via e-mail to LCGCedit@ubm.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.













