Application of SPME Using an Overcoated PDMS–DVB Fiber to the Extraction of Pesticides From Spaghetti Sauce: Method Evaluation and Comparison to QuEChERS
LCGC North America
A solid phase microextraction (SPME) method was developed using a new overcoated PDMS–DVB fiber for immersion extraction of pesticide residues from spaghetti sauce. The overcoating, which consisted of polydimethylsiloxane, offered protection to the SPME fiber, making it more resistant to physical and chemical damage. Also, it increased fiber selectivity for the smaller analyte molecules over macromolecules present in sample matrix. This then allowed it to be used in immersion extraction of a very complex matrix-spaghetti sauce. Performance of the overcoated fiber was compared to a nonovercoated fiber of the same chemistry for method accuracy, precision, and durability. The SPME method developed using the overcoated fiber was then compared to extraction of the same pesticides in spaghetti sauce using a conventional QuEChERS method for extraction and cleanup. SPME was comparable to the QuEChERs method for both accuracy and precision. However, its main advantage over QuEChERS was in sensitivity and method simplicity.
A solid-phase microextraction (SPME) method was developed using a new overcoated polydimethylsiloxane–divinylbenzene (PDMS–DVB) fiber for immersion extraction of pesticide residues from spaghetti sauce. The overcoating offered protection to the SPME fiber, making it more resistant to physical and chemical damage. Also, it increased fiber selectivity for the smaller analyte molecules over macromolecules present in sample matrix. These attributes then allowed it to be used in immersion extraction of a very complex matrix-spaghetti sauce. Performance of the overcoated fiber was compared to that of a nonovercoated fiber of the same chemistry for method accuracy, precision, and durability. The SPME method developed using the overcoated fiber was then compared to extraction of the same pesticides in spaghetti sauce using a conventional QuEChERS (quick, easy, cheap, effective, rugged, and safe) method for extraction and cleanup. SPME was comparable to the QuEChERs method for both accuracy and precision. However, its main advantage compared to QuEChERS was in sensitivity and method simplicity.
Solid-phase microextraction (SPME) is a simple, sensitive, and inexpensive technique that can be used in the extraction of a wide variety of analytes. In addition, it can be considered a “green” chemistry alternative to conventional extraction methods because it does not require the use of solvents and produces minimal laboratory waste. Since its commercial introduction in the early 1990s, SPME has gained acceptance for the quantitative determination of a wide variety of compounds in many different matrices. It can be used to extract analytes by either direct immersion into a sample or exposure to the headspace over the sample. Headspace sampling is the preferred approach because it minimizes fouling of the coating and prolongs fiber life. However, compounds with low vapor pressures, such as many pesticides, will not partition efficiently into the sample headspace, and thus require extraction by immersion. Immersion extraction from samples with very complex and heavy matrices, such as food, presents some challenges to SPME. These types of samples contain a variety of macromolecules, which can remain behind on the fiber after extraction, causing fouling of the coating and shortening fiber life. In addition, any residual material on the fiber after extraction can potentially be transferred to the gas chromatography (GC) system inlet, resulting in contamination of the GC system. A post-extraction wash of the fiber can remove some of this residual material, but for very heavy samples it may not be enough to produce a robust SPME method. These issues have been addressed in a recent development in SPME fiber technology. In recent work published by Silva and Pawliszyn (1,2), an adsorbent SPME fiber was constructed that incorporated an overcoating to make it more amenable to direct immersion into heavy matrices. The overcoating consisted of a 10-µm layer of polydimethylsiloxane (PDMS), which was applied to a 65-µm divinylbenzene (DVB)–PDMS SPME fiber. This procedure resulted in a fiber with a total phase thickness of 75 µm, which was still thin enough to allow for use in a standard 23-gauge needle assembly. An optimized method using this overcoated fiber was then used in the extraction of pesticides from grapes. The researchers found the overcoating to provide enhanced robustness for the PDMS–DVB fiber while providing adequate performance for the application. Most notably, over the course of 130 extractions of grape samples, the overcoating protected the fiber from fouling, and maintained performance with regards to stable pesticide response (1,2).
Most fruits, vegetables, and processed food products represent samples with heavy and complex matrices. These types of samples are commonly analyzed for pesticide residues using the QuEChERS (quick, easy, cheap, effective, rugged, and safe) technique (3,4). Although it is an effective and comprehensive approach, this technique involves the use of solvents and requires several hands-on steps before analysis of the final sample extract. Since the sample is diluted during extraction, a concentration step or large volume injection is often necessary to obtain the required detection levels. In addition, the cleanup step used as part of the QuEChERS methodology provides “just enough” matrix removal, and in the case of very heavy matrices, can result in excess background, which leads to instrument fouling with repeated sample analyses. The use of SPME with the previously described overcoated PDMS–DVB fiber has potential for use with these heavy matrices. The PDMS overcoating provides protection of the DVB, thus making the fiber more robust, and more resistant to fouling from sample matrix. In addition, the chemistry of the PDMS–DVB fiber makes it a good choice for the extraction of many GC-amenable pesticides such as organochlorine, organophosphorus, and triazine classes. In this work, an SPME method was developed using the overcoated PDMS–DVB SPME fiber for the extraction of a variety of pesticides from spaghetti sauce. Spaghetti sauce represents a complex matrix that contains oils, sugars, and pigments. The performance of the overcoated PDMS–DVB fiber was compared to a standard, nonovercoated version of the same fiber chemistry for accuracy, precision, and durability with repeated extractions. Results obtained using the overcoated PDMS–DVB fiber SPME method were then compared to analysis of the same pesticides in spaghetti sauce using QuEChERS for extraction and cleanup.
Experimental
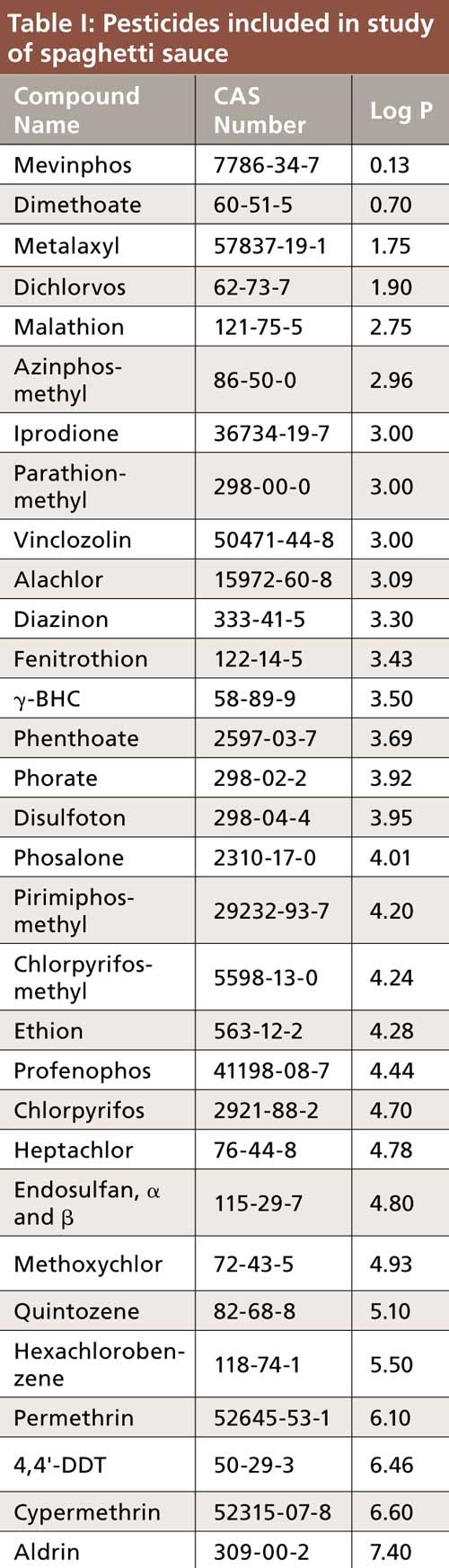
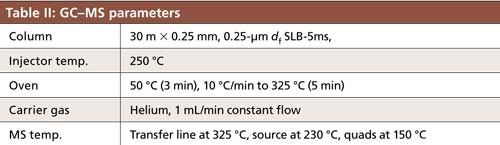
The pesticides used for this study are listed in Table I in order of increasing log P (5,6). Dimethoate and mevinphos have log P values less than 1, indicating their polar nature; thus, it was expected that they would have a low affinity for the PDMS–DVB fiber and would be difficult to extract. Analysis was done on an Agilent 7890B/5977 single-quadrupole GC–mass spectrometry (MS) system operated in selected ion mode (SIM) using the parameters shown in Table II. For each pesticide, one quantitation and two qualifier ions were monitored. The final SPME method was performed on a Gerstel MPS autosampler equipped with an agitator, using the parameters listed in Table III. This method was used for both the nonovercoated and overcoated PDMS–DVB fibers. Optimization of the parameters listed is described later in the text.
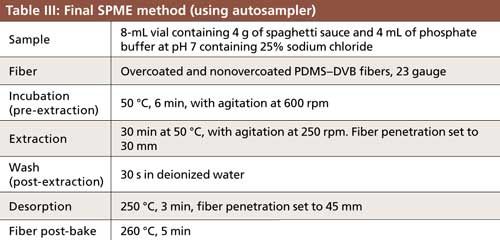
Marinara flavored spaghetti sauce was obtained from a local grocery store. For accuracy, reproducibility, and durability studies, the sample was spiked at 10 ng/g and allowed to equilibrate a minimum of 3 h before analysis. Samples were prepared for SPME by weighing 4 g into a 10-mL vial and adding 4 mL of an aqueous solution containing 25% sodium chloride and 0.1 M phosphate buffer prepared using dibasic sodium phosphate, and adjusted to pH 7 using monobasic sodium phosphate. The resulting sample–phosphate buffer mixture had a pH of approximately 6.
Quantitation of spiked samples was done by external standard calculation against a five-point calibration curve covering a range of 1–20 ng/g, prepared in matrix, and extracted using the SPME method described in Table III. The fiber post-bake listed in Table III was included to prevent carryover. The 260 °C temperature and 5-min post-bake time were chosen based on monitoring fiber blanks run after the highest concentration standard in the calibration curve.
QuEChERS extraction was done using the method described in Table IV. This protocol follows AOAC Method 2007.01, and represents a standard approach for pesticide residue analysis (3). A sample to solvent ratio of 1:1 was used in accordance with the method. To ensure more complete extraction, a mechanical shaker was used. Cleanup was performed using a mixture of primary-secondary amine (PSA), C18, and magnesium sulfate. Both PSA and C18 are sorbents described for use in the QuEChERS technique; with PSA used to remove sugars and acidic interferences and C18 to retain hydrophobic interferences. Analysis was performed by GC–MS-SIM on the same system used for SPME. The GC–MS-SIM conditions were the same as the SPME experiments with the exception of the injection, which was a 1-µL liquid injection into a 4-mm i.d. Focus Liner (MilliporeSigma) with taper. External standard quantitation was done against a five-point matrix-matched calibration curve from 2.5 ng/g to 20 ng/g (25 ng/mL to 200 ng/mL in final extracts) in un-spiked spaghetti sauce extract prepared using the method in Table IV.

Discussion and Results
SPME Method Optimization
During development of the SPME method, the following parameters were found to increase pesticide response and method reproducibility:
- Sample dilution: Diluting the spaghetti sauce with a 25% salt (sodium chloride) water solution improved reproducibility and increased pesticide uptake, thus increasing response.
- Preincubation: Vigorous agitation of the diluted sample for several minutes during this step ensured a homogenous sample–salt water solution during the extraction process.
- Extraction temperature: Using an extraction temperature of 50 °C improved pesticide response and method reproducibility over extraction at 30 °C.
- Post-extraction wash: A post-extraction, preinjection wash of the SPME fiber in deionized water helped to remove residual matrix, thus preventing fouling of the GC inlet, and prolonging fiber life. As shown later in this article, this wash was found to be more effective for the overcoated fiber than for the nonovercoated fiber.
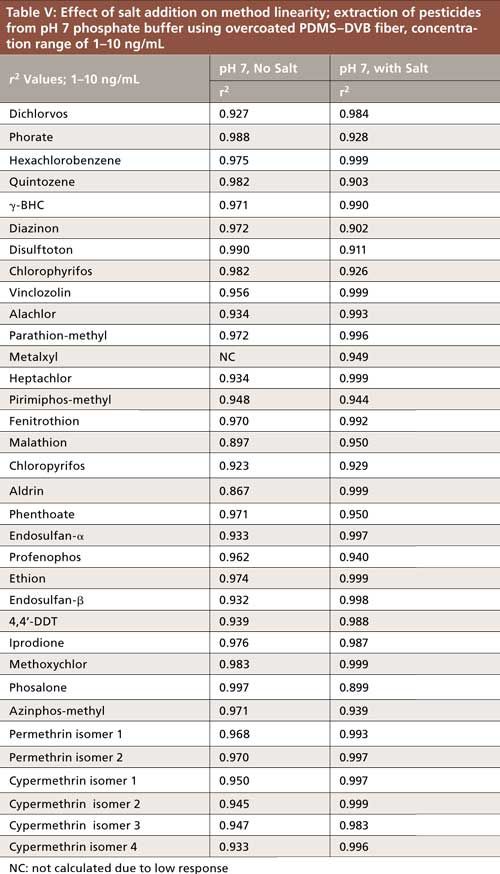
Addition of Salt
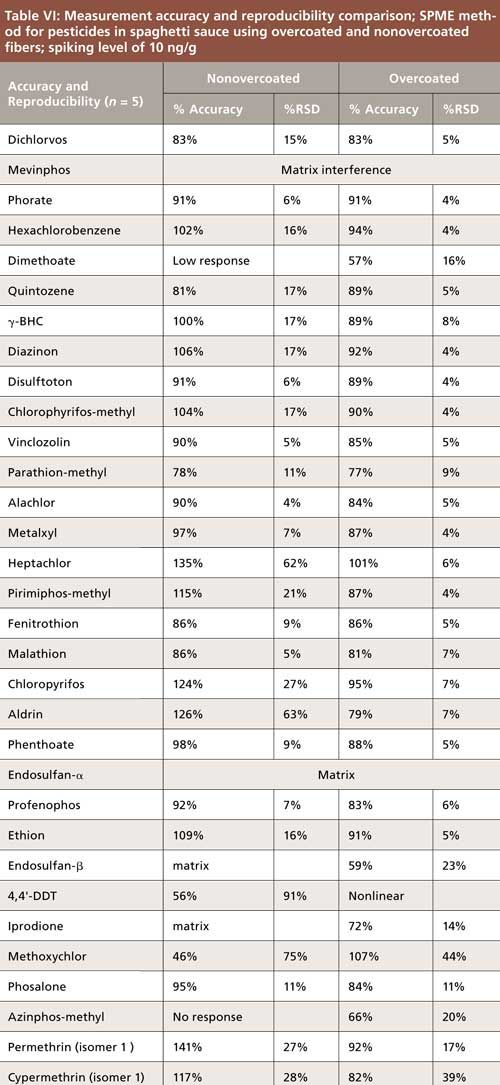
The effect of salt addition was evaluated using spiked phosphate buffer samples at pH 7. Three spiking levels from 1 to 10 ng/mL were extracted at 30 °C using the overcoated PDMS–DVB fiber, with and without the addition of sodium chloride. Salt is used in SPME as a sample additive to increase ionic strength, which shifts analyte equilibrium toward the fiber, thus making analyte uptake more efficient. For this study, a concentration of 25% was chosen as it represents the saturation level of sodium chloride in aqueous solution. The addition of salt improved responses for many pesticides (data not shown). However, its main advantage, as indicated in Table V, was an improvement in linearity for some of the more hydrophobic compounds such as hexachlorobenzene, aldrin, permethrin, and cypermethrin. As predicted initially for dimethoate and mevinphos, low or no response was obtained regardless of salt addition.
Extraction Temperature
The extraction temperature was increased from 30 °C to 50 °C to improve reproducibility of the pesticide response obtained from the spaghetti sauce matrix. Because an extraction temperature of 30 °C produced adequate linearity and response from a salt–phosphate buffer solution, it was used initially for extraction of the spaghetti sauce; however, measurement reproducibility was poor. Increasing the extraction temperature to 50 °C (with all other parameters the same) resulted in increased response for many of the target compounds from the spaghetti sauce, and significantly better reproducibility. These improvements are illustrated in Figures 1 and 2. Figure 1 shows a comparison of the average responses obtained from sets of spaghetti sauce samples spiked at 10 ng/g, and extracted at 30 °C and 50 °C. The higher extraction temperature resulted in an increase in response for most of the target compounds. Figure 2 shows a comparison of reproducibility as percent relative standard deviation (%RSD) values for the two sample sets. The %RSD values obtained for the average responses are plotted on the y-axis. A significant decrease in %RSD is shown from 30 °C to 50 °C, indicating an improvement in reproducibility of the SPME method with the higher temperature. The higher extraction temperature could be helping the SPME process in several ways. First, it may be increasing kinetics of the extraction, resulting in faster analyte uptake. Disruption of binding between the pesticides and the sample matrix may also be occurring, which will produce more free analyte for extraction by the fiber. Finally, the higher temperature could be increasing the water solubility of the more hydrophobic pesticides, thus making them more readily available for extraction. Additional studies are needed to gain a better understanding of these effects.
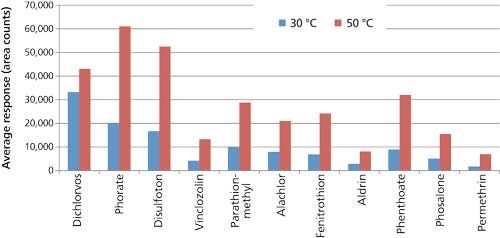
Figure 1: Effect of extraction temperature on pesticide response from spaghetti sauce using the overcoated fiber (10 ng/g, n = 3).
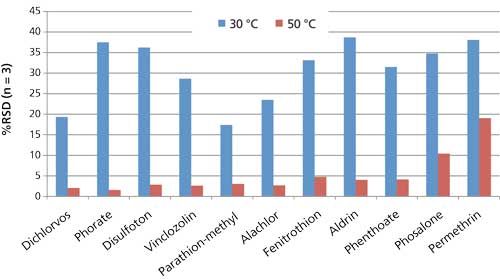
Figure 2: Effect of extraction temperature on measurement reproducibility of pesticides extracted from spaghetti sauce using the overcoated fiber (10 ng/g, n = 3).
Accuracy and Reproducibility
A set of spiked replicates of spaghetti sauce (n = 5) was extracted using both the overcoated and nonovercoated PDMS–DVB fibers, and accuracy and precision results are summarized in Table VI. Accuracy was similar between the two fibers for many of the pesticides; however, reproducibility was significantly better with the overcoated fiber. As indicated in Table VI, several pesticides could not be quantitated because of matrix interference. 4,4′-DDT did not exhibit linear response using the overcoated fiber, and showed erratic and decreasing response with the nonovercoated fiber as the runs progressed. Pesticides showing the most reproducible responses using both fiber types tended to be those with log P values less than 4.5, although there were several exceptions. Dimethoate, despite having a log P value less than 1, exhibited some response from the spaghetti sauce matrix; however, its response was too low to quantitate using the nonovercoated fiber. Response was better using the overcoated fiber; however, accuracy was <60%. The response of mevinphos from the spaghetti sauce could not be evaluated on either fiber because of matrix interference. The organochlorine pesticides heptachlor, aldrin, 4,4′-DDT, and methoxychlor exhibited very high %RSD values using the nonovercoated fiber. This was due to rapidly dropping responses from the first to last sample run, most likely resulting from contamination of the GC inlet. This behavior was also observed as part of the durability sequence, which is described later in this article.
Durability
Direct immersion of an SPME fiber into a heavy matrix such as spaghetti sauce can leave residual sample on the fiber, and thus a wash step should be done before desorbing the fiber in the GC injection port. The overcoating on the PDMS–DVB fiber was expected to extend fiber life by preventing matrix from sticking to the fiber, and making the wash step more effective. Fiber durability was studied for the overcoated and nonovercoated PDMS–DVB fibers by doing repeated analyses of spiked spaghetti sauce samples while monitoring pesticide response. The analyses were done as a continuous, uninterrupted sequence of 30–40 samples. Spiked salt–phosphate buffer samples were run as controls at the beginning, middle, and conclusion of each test sequence. Fiber blanks were also run throughout the sequence to monitor for carryover. The spiked spaghetti sauce used for the testing was prepared in bulk, mixed for 1–2 h on a tumbler, and allowed to equilibrate overnight before testing.
To study change in response from beginning to end, area count responses were plotted as values normalized to run 1 in each test sequence. Thus, with run 1 having a value of “1.0,” subsequent values of <1 represent a drop in response, and >1 an increase in response. Overall, the overcoated fiber exhibited a more steady response throughout the course of 38 sample runs. The nonovercoated fiber showed a decline from 1 to 20 samples and then a drop to zero response thereafter. An example of this behavior is shown in Figure 3 for methyl parathion. The spiked buffer run at the end of the test sequence for the nonovercoated fiber also showed total loss of response for all analytes. Examination of the SPME fiber and GC system showed that the fiber was still intact; however, dark residues were observed in the inlet liner and in the first few inches of the GC column. System maintenance (liner change and clipping one loop of column) restored pesticide response, indicating that instrument contamination was the cause of the dramatic response loss observed with the nonovercoated fiber. This instrument contamination did not occur when using the overcoated fiber, which indicates that the post-extraction wash step was not as effective when using the nonovercoated fiber. Thus, more matrix was retained on the nonovercoated fiber and introduced into the inlet and column during injection.
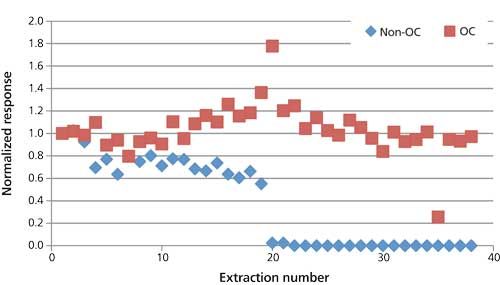
Figure 3: Comparison of parathion-methyl response from spiked spaghetti sauce with repeated extractions using the overcoated (OC) and nonovercoated (non-OC) fibers.
Visual comparisons of the overcoated and nonovercoated fibers after the test sequence are shown in Figures 4 and 5. Before use, both fibers appeared white in color. After the test sequence, the overcoated fiber showed some carbon buildup (visible as dark coloration), but was still white in color. The nonovercoated fiber was brown in color and completely covered in caramelized residue. As indicated in Figure 5, some physical damage was sustained by the nonovercoated fiber as coating pulled away from the crimp portion of the fiber assembly. This type of damage was not evident on the overcoated fiber.

Figure 4: Overcoated (OC) and nonovercoated (non-OC) fibers after extraction of 38–40 spaghetti sauce samples.
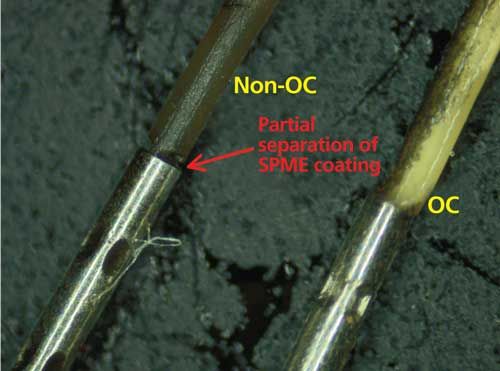
Figure 5: Overcoated (OC) and nonovercoated (non-OC) fibers after extraction of 38–40 spaghetti sauce samples; crimp portion of the SPME fiber assembly.
Comparison to QuEChERS
QuEChERS is a very widely accepted method for extraction and cleanup of pesticide residues from different foods. The specifics of the method, as described in AOAC Official Method 2007.01 and EN 15662, describe a two-step process; first an extraction with acetonitrile followed by salting out, and then a cleanup step using bulk sorbents such as C18 and PSA. The resulting extract is then analyzed by GC–MS or liquid chromatography–mass spectrometry (LC–MS) following concentration or solvent exchange. For pesticides analyzed by GC–MS, SPME offers the potential of simplifying the process by essentially combining the extraction and cleanup steps, and eliminating the need for a concentration step. In addition, with the advent of X-Y-Z autosamplers, the SPME process can be easily automated.
In Figures 6 and 7, the accuracy and reproducibility results obtained for the set of spiked spaghetti sauce samples prepared using QuEChERS are compared with those from the SPME method using the overcoated fiber. Accuracy was determined as a percentage of the amount of pesticide measured versus the amount spiked. In the QuEChERS extracts, diazinon, malathion, and azinphos-methyl could not be analyzed because of matrix interferences.
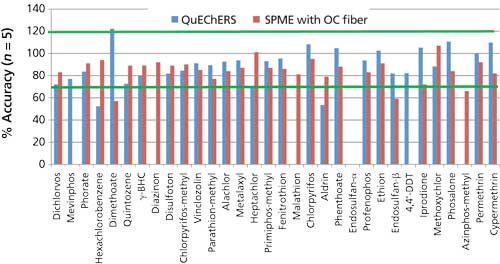
Figure 6: Comparison of method accuracy: QuEChERS versus SPME using an overcoated fiber. Green bars indicate the 70–120% recovery range generally considered acceptable in pesticide residue analysis.
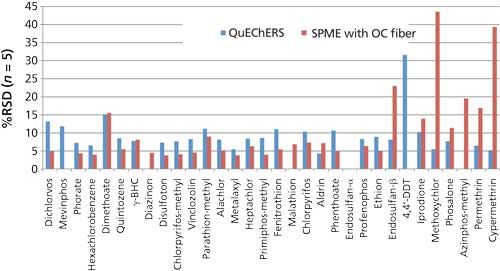
Figure 7: Comparison of method reproducibility (as %RSD), QuEChERS versus SPME with the overcoated (OC) fiber.
Some of the findings from the comparison of the QuEChERS and SPME methods were as follows:
- There was more matrix interference in the QuEChERS samples than the SPME samples. This interference was visible for some pesticides when reviewing data in the extracted ion chromatogram (EIC) windows. This effect is illustrated in Figure 8 for alachlor, quantitated using m/z = 160.
- Accuracy was comparable between the techniques for many pesticides, with most compounds in the generally acceptable range of 70–120%, indicated with green lines in the bar chart in Figure 6. Accuracy was better for several organochlorine pesticides (hexachlorobenzene, aldrin, and so forth) using SPME. The spaghetti sauce contained 3% fat, and it has been reported when doing QuEChERS that the presence of fat can reduce the extraction efficiency of highly lipophilic pesticides into acetonitrile (6,7). In addition, reduced recoveries of these pesticides due to retention on the C18 cleanup sorbent cannot be ruled out.
- Reproducibility is shown in Figure 7 as %RSD for each pesticide. Pesticides are indicated on the x-axis in elution order. For pesticides eluted up through ethion, reproducibility was slightly better with SPME, as indicated by the lower %RSD values. For the heavier pesticides eluted later than ethion, (endosulfan-beta onward), reproducibility was better using QuEChERS.
- Overall sensitivity of the SPME method was better than the QuEChERS method. This was indicated by the need to concentrate the QuEChERS extracts before GC–MS-SIM analysis.
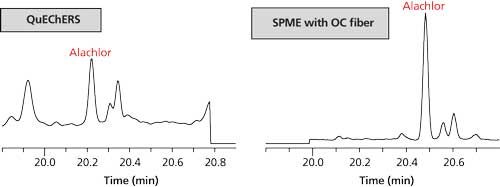
Figure 8: QuEChERS extract and SPME overcoated (OC) fiber analysis of spaghetti sauce; extracted ion window of quantitation ion for alachlor (m/z 160). The difference in retention time is because of system maintenance done before analysis of the QuEChERS extracts.
The ruggedness of the QuEChERS method was evaluated in a similar manner to the SPME durability test by injecting spiked spaghetti extract a total of 32 times into the GC–MS system and monitoring pesticide response. The resulting data was then compared with the durability data obtained previously using the overcoated SPME fiber method. The QuEChERS extract was expected to contaminate the GC–MS system much more quickly than the overcoated-fiber SPME method; thus it was predicted that pesticide responses would decline more rapidly. For comparison, as with the SPME durability data, responses were normalized to the first run in each test sequence. Organophosphorus pesticides showed a more rapid decline in response using QuEChERS than SPME, while response of the organochlorine pesticides declined at similar rates between the two techniques. Examples of this decline for an organophosphorus pesticide, methyl parathion, and an organochlorine pesticide, hexachlorobenzene, are illustrated in Figures 9 and 10, respectively. Organophosphorus pesticides are generally more susceptible to adsorption on active sites in the GC inlet than organochlorine pesticides. Residue was observed in the quartz wool of the liner after injecting the liquid samples. Thus, it is possible that the more rapid decline of the organophosphorus pesticides during injection of the QuEChERS versus the SPME samples was caused by quicker deposition of residue in the GC inlet.
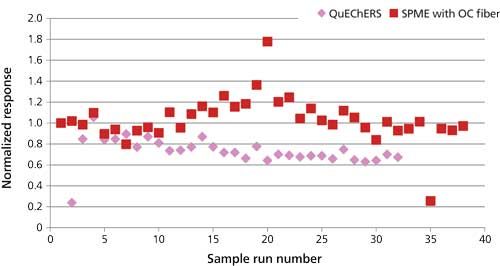
Figure 9: Comparison between QuEChERS and SPME method with overcoated (OC) fiber. Parathion methyl response with repeated injections–extractions of spiked spaghetti sauce.
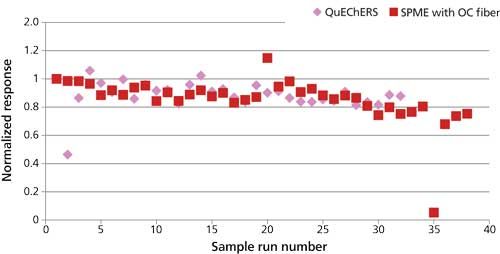
Figure 10: Comparison between QuEChERS and the SPME method with the overcoated (OC) fiber. Hexachlorobenzene response with repeated injections–extractions of spiked spaghetti sauce.
Conclusions
In this study, a solid-phase microextraction method was developed for the extraction of pesticide residues from spaghetti sauce. The addition of salt and use of an elevated extraction temperature of 50 °C contributed significantly to increased pesticide response and improved method reproducibility. The overcoated PDMS–DVB fiber showed an advantage compared to a standard, nonovercoated fiber with regards to reproducibility and durability. For accuracy, performance of the two fiber types was similar. Fiber durability when immersed into the heavy spaghetti sauce matrix was extended through a post-extraction wash step, and this step was found to be more effective with the overcoated fiber than the nonovercoated fiber.
Compared to QuEChERS, the SPME method using the overcoated fiber showed an advantage with lower background overall, and better accuracy for several organochlorine pesticides. GC method ruggedness was better for the organophosphorus pesticides using SPME, and similar for the organochlorine pesticides. The SPME method was more sensitive than the QuEChERS method, as was evidenced by the need to concentrate the QuEChERS extracts before analysis. In addition, the SPME method did not require the use of solvents, was easier to perform, required less “hands-on” sample preparation time, and produced less laboratory waste than the QuEChERS method.
With the development of overcoated fibers, immersion SPME can now be extended to matrices that were previously considered too complex and heavy for this technique. As this study shows, SPME with overcoated fibers has potential to be considered for use as a complementary technique to conventional forms of extraction, such as QuEChERS, in the analysis of pesticides and other contaminants from food matrices.
References
- E.A. Souza-Silva and J. Pawliszyn, Anal. Chem. 84, 6933–6938 (2012).
- E.A. Souza-Silva and J. Pawliszyn, J Agric. Food Chem. 63(18), 4464–4477 (2015).
- AOAC Official Method 2007.01, “Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate.”
- European Standard EN 15662, "Foods of Plant Origin-Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extraction/Partitioning and Cleanup by Dispersive SPE-QuEChERS-Method" (2008).
- The Pesticide Manual, A World Compendium, 13th edition, C.D.S. Tomlin, Ed. (BCPC, Hampshire, UK, 2003).
- Compound Properties viewer, available at www.chemicalize.org.
- S.C. Cunha, S.J. Lehotay, K. Maštovská, J.O. Fernandes, M. Beatriz, and P.O. Oliveira, J. Sep. Sci.30, 620–632 (2007).
- S.J. Lehotay and K. Maštovská, J. AOAC Int.88(2), 630–638 (2005).
Katherine K. Stenerson is a principal scientist with MilliporeSigma in Bellefonte, Pennsylvania. Tyler Young is a Research & Development (R&D) intern with MilliporeSigma. Robert Shirey is a principal scientist with MilliporeSigma. Yong Chen is a senior scientist with MilliporeSigma. Leonard Sidisky is a senior manager in Research & Development with MilliporeSigma. Direct correspondence to: katherine.stenerson@sial.com

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).






