Meeting Review: Latest Advances in the Analysis of Complex Environmental Matrices, 2019
The Column
A biennial meeting jointly organized by the Environmental Chemistry Group, Separation Sciences Group, and the Water Science Forum, and discussing the latest advances in the analysis of complex environmental matrices, is now in its eighth year. The most recent iteration of the event occurred on Friday 22 February 2019 in the Science Suite, Royal Society of Chemistry, Burlington House, in London, UK. This meeting review offers an overview of what is happening in the industry.
Liliia/stock.adobe.com

A biennial meeting jointly organized by the Environmental Chemistry Group, Separation Sciences Group, and the Water Science Forum, and discussing the latest advances in the analysis of complex environmental matrices, is now in its eighth year. The most recent iteration of the event occurred on Friday 22 February 2019 in the Science Suite, Royal Society of Chemistry, Burlington House, in London, UK. This meeting review offers an overview of what is happening in the industry.
The application of analytical chemistry is common within a large number of disciplines and subject fields. Each area of the application has its own particular challenges concerning sample complexity, treatment, and analysis.
Over the last eight years, the Environmental Chemistry Group (ECG), with the Separation Science Group (SSG) of the Royal Society of Chemistry (RSC), has sought to present the latest applications of new and developing analytical techniques to complex samples within the area of environmental chemistry.
There are a number of unique challenges within this field and the sheer complexity of the sample, coupled with low analyte concentration, often requires the implementation of novel analytical strategies. If we couple matrix complexity with an important variable within environmental research, spatial sampling, we have a further difficult requirement. This is the recording of not only the type of sample, but also its three-dimensional location within the environment. If we consider that each experiment may be performed over a long period of time using multiple time points, locations, and depths, we can begin to see that large numbers of often uniquely complex samples and data can be generated.
The latest meeting, the fourth of the biennial event, took place on Friday 22 February 2019 at the RSC, Burlington House, in London, UK. The meeting was well attended by over 60 delegates. The joint organizers of the meeting, the Environmental Chemistry Group (Roger Reeve), the Separation Science Group of the RSC-Analytical Division (Lee Williams), and the Water Science Forum (Graham Mills), developed a programme to show the advances within each stage of the major workflow of the discipline; sample collection, sample preparation and clean-up, final analysis, and the ever increasingly important stage of chemometric data analysis. Machine learning, data treatment, and statistical analysis of complex matrices has been seen in a number of fields, such as metabolomics and diagnostic healthcare, for a number of years and the application and benefits to the environmental field is becoming more recognized and widespread.
The first of the presentations on environmental sampling was given by Anthony Buchanan (SepSolve Analytical, UK) with a lecture on the “Enhanced Confidence in River Water Quality Monitoring Using Passive Sampling and GC×GC–TOF-MS (Two-Dimensional Gas Chromatography, Time-of-Flight Mass Spectroscopy) with Tandem Ionization”. Perhaps obviously, the role of routine environmental monitoring is to ensure the quality of the environment and provide safety to those within it. Evaluating a particular location using grab or instantaneous sampling may miss important pollution events and, therefore, the use of long-term passive sampling may be more suited. Current challenges for this type of analysis include new “priority” substances and emerging pollutants.
A silicone rubber passive sampler was used to collect and concentrate hydrophobic substances in this investigation with four weeks sampling time, on a river system at eight different locations.
Analysis was performed using twoâdimensional gas chromatography, a tool that is becoming more common in complex sample analysis. Flame retardants, fragrance bases, and pharmaceuticals were detected. A novel approach to sample identification was implemented using fast switching between high eV and softer, low eV ionization voltage in the ubiquitous electron ionization (impact) source. This produced two MS datasets from a single run with no added analysis time, but extended the quality of the mass spectra and ability to retain more of the molecular ion to aid in compound identification.
The limitations of grab sampling were a theme further developed by Gary Fones (University of Portsmouth, UK) with his talk: “Can Passive Sampling Devices Provide More Useful Data than Discrete Samples?”
Passive samplers offer long-term monitoring, identifying pollution trends, time frames, and patterns, and can locate sources of pollution with more robust time integrated data than instantaneous sampling. Grab sampling provides only a “snapshot” of the pollution situation (Figure 1) and may not be representative of occasions where concentrations of individual pollutants fluctuate or where the overall composition may not be homogeneous.
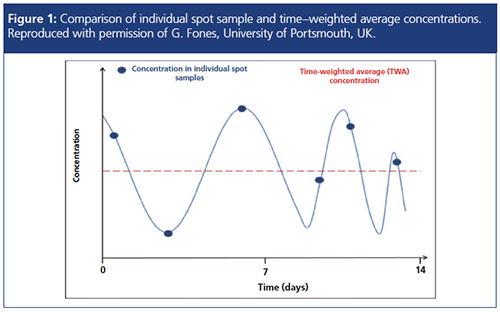
A passive sampling device, designed to monitor a wide variety of pollutants in a range of aqueous environments, was chosen for the analysis of polar pesticides in the River Rother catchment system (West Sussex, UK). A total of 14 sites and a twoâweek continuous sampling time was chosen for the study. The analysis was performed using liquid chromatography quadrupole timeâofâflight mass spectrometry (LC–QTOFâMS). The advantages of QTOF, namely a high specificity, sensitivity, accurate mass, and large dynamic range, provided the ability to consistently identify and determine the relative levels of 51 compounds within the river system. The data demonstrated that the concentrations of pollutants was in flux within the sampling period, something that would not have been evident within a single time-point sample.
An additional approach to long-term monitoring was presented by Alistair Boxall (University of York, UK) in “Temporal and Spatial Variations of Pharmaceutical Contamination in an Urban River System”. This research took point samples at 11 sites entering the River Ouse system (Yorkshire, UK) monitoring 41 pharmaceuticals using repeated grab, rather than passive, longâterm sampling. This built up a detailed model through which it was possible to predict time-based variations of individual pollutant concentrations at each section of the river system. It was noted throughout this study that pollutants decreased significantly, sometimes to effectively zero, depending on the substrate sampled, in particular, those substrates that were washed away during storms. The model could be successively upgraded as more local details of the river system are introduced. Monitoring of pharmaceuticals in this way has been extended to a global network and subsequent correlation of the results suggests a greater problem of high concentrations in lower income countries.
The use of multiple point samples rather than long-term passive sampling was described through the lecture by Katie Read (National Centre for Atmospheric Science, NCAS, University of York, UK), who introduced the challenges and implications of the use of “GC–TOF for Remote Monitoring-Cape Verde Atmospheric Laboratory”.

Using remote, automated equipment (Figure 2) posed many challenges when setting up the distant laboratory and much of the talk discussed additional problems of atmospheric analysis in a laboratory located in the midst of the Atlantic. Atmospheric dust, salt crystal formation, sea water, and abrasive particles of volcanic rock each contributed to enhanced corrosion on the instruments. The air masses passing the laboratory were from a variety of origins and specific effects could be observed from those arriving from Africa, Europe, and North America, in addition to pristine Atlantic air. Despite this, both in situ measurements and sampling with subsequent laboratory analysis were used to monitor air samples.
The results of the comparison between existing GC–MS and new GC–TOF-MS analytical instruments demonstrated a reduction in the sensitivity gap between quadrupole and triple quadrupole systems over QTOF mass analyzers. Results from the laboratory suggest that 50% more ozone is destroyed in this region than was predicted by the climate models as a result of the catalytic destruction by natural halogens in sea spray.
In addition to automated sampling, automatic sample preparation was discussed by John Quick (ALS Environmental Ltd.), who presented “Exploring the Advantages of Automated Sample Preparation and GC–TOFâMS for Semivolatile Organic Compound and Pesticide Analysis in Environmental Waters”.
Environmental samples are frequently large volumes, hundreds of millilitres compared to the tens or sub-millilitre quantities found in clinical or pharmaceutical samples. The requirements of analyte extraction and preconcentration before analysis can lead to laborious and time-consuming sample preparation. Using automation in this process provides advantages in terms of increased method accuracy and precision and the ability to use lower sample volumes through more efficient processes and the reduction of consumables.
In the analysis of alkylphenols and ethoxylates, the sample was simultaneously extracted and derivatized. In a second example, automated use of liquid–liquid extraction in a dispersive liquid–liquid microextraction format was used. This method offered time and sample saving over standard liquid–liquid and solid-phase extraction (SPE) techniques.
As is ever the case in progressive measurement science, where data can be collected both rapidly and in increasingly larger quantities, the use of advanced chemometrics to perform data mining experiments, prediction of analyte properties, and characterization of unknown compounds has led to an ever increasing uptake of this approach within environmental research.
This was first demonstrated in the presentation by Leon Barron (King’s College London, UK), whose lecture on “Mixing High Resolution Chemical Analysis and Machine Learning in Ecotoxicology for Aqueous Invertebrates” sought to demonstrate new protocols using new and existing tools for the analysis of unknown chemical entities.
Samples were taken from water, fish, and invertebrates. The samples were analyzed, first in a targeted approach, using LC tandem mass spectroscopy (MS/MS) allowing characteristic fragments to be recorded. Suspect compound screening was undertaken by LC–high-resolution mass spectroscopy (HRMS) to provide a higher level of mass accuracy, which greatly aids the identification of unknown analytes.
The use of machine learning provided a good predictive model of retention time on LC stationary phases and was further developed to bio-concentration factors
This was the first of two lectures highlighting the use of computational techniques in chromatographic data analysis to aid compound identification, prediction of chemical properties, and possible environmental effects. The keynote speaker, Emma Schymanski (Luxembourg Centre for Systems Biomedicine, Luxembourg), illustrated further with her presentation on “Environmental Informatics to Identify Unknown Chemicals and Their Effects”, which discussed the number of European, US, and worldwide community initiatives to help connect chemistry and toxicity with environmental observations.
Within the work, 364 targets were identified in Swiss wastewater. Targeted, suspect, and nontarget screening is required to attempt to identify the number of unknown compounds within the environmental system. Compound characteristics can be built using a number of known compounds (targeted) and confirmed in a number of available databases. Suspect screening involves selection of candidates based on known and calculated physical properties. The implementation of a workflow involving several mass spectral libraries was used in an attempt to identify compounds, as many libraries, whilst overlapping, had unique entries.
It was noted by many attendees that one of the most impressive aspects of the work was the level of cooperation and data exchange between research groups. The NORMAN network within the EU exchanges information on emerging environmental substances, their validation, and harmonization of analytical techniques. In this investigation the NORMAN suspect list exchange and its chromatography data bank (digital sample freezing platform) were used and have been made freely accessible to anyone who requires it. In this way the more the algorithms and databases are enhanced and added to, the greater the probability of compound identification.
The final theme of the event, moving from identification of unknown substances, was the characterization of the specific pollutants in environmental matrices.
The first of these was a presentation by Caroline Gauchotte-Lindsay (University of Glasgow, UK) on “Micro- and Nano-Plastic Pollution of Freshwater and Wastewater Treatment Systems”. Her group has been attempting to investigate not only the range of physical size, from nano- to micro-scale, that exist within environmental contamination by plastics, but also the different morphologies present.
The demonstration of contamination of marine organisms and water samples by nanoparticles has, in recent years, been elevated from academic research articles and conferences to the forefront of discussion within the general media and is a concern for public health and the marine environment.
Gauchette-Lindsay showed that nanoparticle contamination can be fibrous as well as the more expected, solid bead-type. These different morphologies may change how the pollutant behaves in the environment.
After pretreatment, sediments were investigated by scanning electron microscopy (SEM) coupled with energy dispersive spectroscopy (EDS). Fibres were found to comprise approximately 88% and 95% of all plastic pieces. Characterization of water samples was undertaken by Fourier-transform infrared attenuated total reflectance spectroscopy. It was further demonstrated that fibres were predominantly plastics (58% polypropylene and 5% polyethylene), the rest being cellulose (11%) or of unknown composition.
The final session of the day was a presentation by Wai-Chi Man (Thermo Fisher Scientific, UK) on the “Power of Ion Chromatography with Mass Spectrometry for Polar Pesticides in Water”. Ion chromatography (IC) has often been thought of as a specific technique used only for the analysis of inorganic ions. However, she showed that the method was more than this single use strategy and was suitable for the separation of highly polar species as well as ionic species in samples.
Polar pesticides in both food and environmental samples were determined at levels well below US Environmental Protection Agency regulatory limits without chemical derivatization using IC–MS, and the technique itself was shown to have a very low chemical noise, thereby reducing interference.
The presentation closed by posing a question based upon a conversation about a separation that had been developed, but which reaches to the core of analytical science; whether the user wants a slow separation of all possible components, or a more rapid analysis where there was peak overlap of the main analyte of interest with other species.
The answer depends, of course, on the application, but reminded us all of the multiple and sometimes conflicting choices needed in deciding the most appropriate analytical technique.
Acknowledgements
This article was written by Lee Williams, Roger Reeve, and Graham Mills based on synopses prepared at the event and additional information from presentations and slides supplied by the speakers. We gratefully acknowledge the industry meeting sponsors: LECO UK, JSB, SepSolve Analytical, and Shimadzu UK. Financial support is also acknowledged from the Environment Sustainably and Energy Division of the RSC.
Lee Williams is senior lecturer in analytical chemistry at the University of Sunderland. His interests are the analysis of compounds in highly complex matrices using hyphenated techniques and the use of chemometrics to provide robust statistical validation.
Roger Reeve has recently retired from the University of Sunderland where he was senior lecturer. His interests, are instrumental analysis of environmental pollutants and the behaviour of pharmaceuticals in the environment.
Graham Mills is professor of environmental analytical chemistry at the University of Portsmouth. His interests are measuring pollutants in water using novel monitoring devices combined with chromatographic and mass spectrometric analytical techniques. He was the inventor of the passive sampler described in this article.
E-mail: lee.williams@sunderland.ac.uk / rgrreeve@gmail.com /graham.mills@port.ac.uk
Website: www.rsc.org/Membership/Networking/InterestGroups/Environmental/

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.











