Meeting Review: Emerging Separation Technologies 2019
Organized by The Chromatographic Society and the Separation Science Group of the Royal Society of Chemistry’s (RSC) analytical division, the Emerging Separation Technologies meeting was held on the 28 March 2019 at the RSC’s central London headquarters in Burlington House.
liuzishan/stock.adobe.com
Organized by The Chromatographic Society and the Separation Science Group of the Royal Society of Chemistry’s (RSC) analytical division, the Emerging Separation Technologies meeting was held on the 28 March 2019 at the RSC’s central London headquarters in Burlington House.
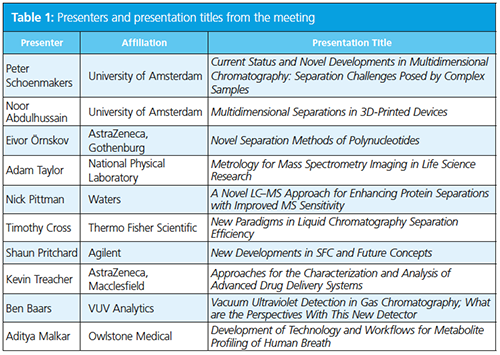
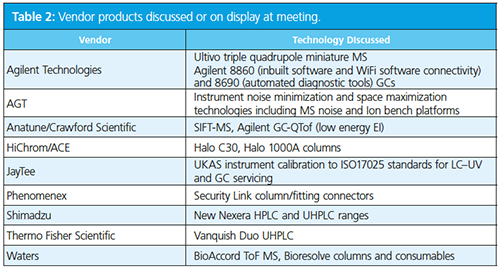
The third Emerging Separation Technologies meeting organized by The Chromatographic Society and the Royal Society of Chemistry’s (RSC) analytical division separation science group was held at the historic Burlington House near Piccadilly, in London, UK. The meeting was opened by one of the principal organizers, Adrian Clarke of The Chromatographic Society, who welcomed the attendees and thanked the vendors for their continued support of the Society’s meetings, which enabled international scientists to present. He discussed that the meeting series focuses on “new instrumentation, applications, and analytical approaches” while also “showcasing emerging scientific professionals”. The programme (Table 1) was well balanced and, as things transpired, had appropriate timings to allow valuable discussion at the end of each talk. A list of vendors in attendance is listed in Table 2.
Clarke chaired the first session and introduced Peter Schoenmakers of the University of Amsterdam, who discussed the many areas of research in his group focusing on approaches for the separation of complex sample types. His talk “Current Status and Novel Developments in Multidimensional Chromatography: Separation Challenges Posed by Complex Samples” featured much of his latest work on multidimensional chromatography. His first example illustrated the separation of ethylene oxide-propylene oxide polymers using hydrophilic interaction liquid chromatography (HILIC) × reversedâphase liquid chromatography (LC). HILIC was used to separate different polar head groups in the first dimension while reversed-phase LC separated the analytes by hydrophobicity in the second dimension. He showed a second example of castor oil-derived polyethylene oxide (PEO) where all polar end groups were identified (1).
Schoenmakers then moved on to how chromatography can help characterize higher order polymer structures for both synthetic and biological species. He showed how size-exclusion chromatography (SEC) or hydrodynamic chromatography (HDC) can be used in combination with field-flow fractionation (FFF) to do this. Multiple detectors can be used to help with this, for example, UV, refractive index (RI), viscometry, and multi-angle light scattering (MALS). An interesting observation was that higher salt concentrations in the mobile phase can lead to an increased number of higher order structures. This was exemplified by the identification of β-galactosidase dimers of 200 kDa molecular weight (MW) from Kluyveromyces yeast. He also discussed the analysis of polymeric nanoparticles using HDC × SEC, which can provide information on MW and particle size in a single analysis (2).
He then moved back onto multidimensional chromatography of biofluids using this approach to identify steroids in bovine urine (3). He discussed his group’s work with Syngenta looking at naturally occurring surfactants using normal-phase LC × reversed-phase LC, which separated ~4800 species and equated to approximately 1 peak per second over the course of the run. He also highlighted his group’s work developing in silico approaches to twoâdimensional (2D) method optimization (the “PIOTR” project), which he said allows methods to be optimized in days rather than months (4).
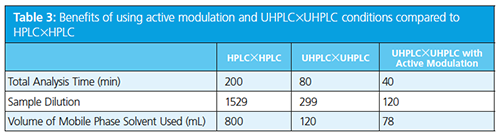
In the next part of Schoenmakers’ talk he discussed issues with coupling different types of separation and the importance of the interface; for example, if nonpolar and polar mobile phases are combined they will de-mix leading to poor chromatography in the second dimension. This issue could be alleviated through the use of sample trapping in place of a sample loop in the interface, an action he called active modulation. He compared these to a high performance liquid chromatography (HPLC) × HPLC separation and showed many benefits of this approach in terms of analysis time, sample dilution (and therefore method sensitivity), and mobile phase usage (Table 3).
Schoenmakers then discussed how the modulator loops could be replaced with reactors to promote different types of controlled degradation. Samples can be parked and left to react for several minutes before transfer onto the second dimension. He exemplified this in a reversed-phase LC × ion-exchange chromatography (IEC) separation with an immobilized enzyme reactor (“IMER”), which has a zigzag channel for increased surface area. This approach reduced the total analysis time per sample down from 22.5 h to 4 h (5). Another example was the analysis of ethanol-derived sugars in beer where the three-dimensional (3D)-printed reactor included microbes (immobilized microbe reactor-“IMMER”). For the final example he described how the loop was replaced with a light cell for photoâcatalyzed degradation in a project called “TOOCOLD”. Analytes were parked in the cell and stressed photolytically. This approach has been used to understand synthetic dye degradation in paintings in work sponsored by Amsterdam’s Van Gogh museum.

The second talk in the session was from Noor Abdulhussain (Figure 1), who is one of Professor Schoenmakers second year doctoral students at the University of Amsterdam. Her presentation focused on the potential of 3D-printed structures for multidimensional chromatography in a project termed “STAMP” (Separation Technologies For a Million Peaks). She started her talk by stating that resolution in multiple dimensions (spatial) is not a new concept and was first described in 1948 (6). The use of cubic separation approaches to generate high peak capacities was also described by Guiochon in 1983 (7) and more recently by Davydova (8). To generate a peak capacity of 125000, around 10000 channels are required on 3D structure.
She described three approaches to making 3D-printed separation structures:
- Digital light processing (DLP) using materials such as Teflon, which produces 200–400 µm separation channels (9). This approach produces high resolution devices but has limited solvent compatibility.
- Selective laser melting (SLM), which uses metal powders such as aluminium, titanium, or steel producing channels of 100–150 µm (10). This produces robust devices but is expensive.
- Fused deposition modelling (FDM), which uses ceramics or polyamide. This approach provides quick prototyping with a variety of materials but has some pressure limitations.
The 3D device she is building utilizes isoelectric focusing (IEF) in the first dimension, which uses a pH gradient and molecules migrate under the influence of an electric field to the pH region where it becomes neutral. At various parts of the fabricated channel, channels to the second dimension are placed. The viability of this device was tested and proved using a pH indicator dye, which separated each coloured component at the correct pH region. She also demonstrated the separation of ribonuclease A from bovine pancreas. She noted that different filament types used for printing the devices had different physical and separation properties.
In the second dimension, a reversedâphase column approach was fabricated. The monoliths were first fabricated using BuMA-EDMA polymers, but liquid leaks were observed as a result of inter-channel voids. When this was replaced with black polypropylene, this issue was prevented. To move the samples between channels, a novel “twisting” mechanism was fabricated to prevent channel leakage (11).
Approaches towards better characterization of biopharmaceuticals are becoming increasingly important as pharmaceutical companies move from smaller to larger molecule development portfolios. After the break, Eivor Örnskov from AstraZeneca discussed approaches to analyze polynucleotides, including antisense oligonucleotides (ASO) and messenger RNA (mRNA), in her presentation “Novel Separation Methods of Polynucleotides”. Örnskov described how ASOs are fabricated on solid supports and each step adding an additional nucleotide can lead to additional impurities. The longer the oligo, the more impurities, and for standard oligos, these are often removed using preparative chromatography. After purification the number of impurities is in the order of 30 to be analyzed. In addition, these are regulated under small molecule guidelines requiring many of the impurities to be quantified and identified (typically >0.05% area/area). LC methods available today cannot reach those levels, therefore reporting limits 0.2% area/area are applied.
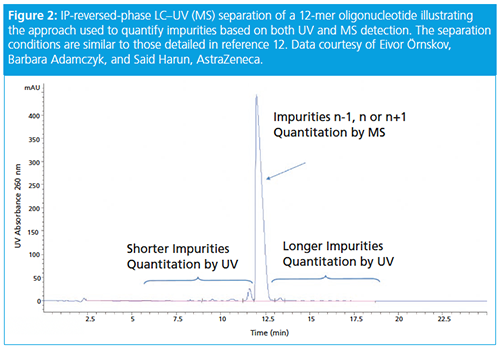
ASO chromatographic methods are largely based on (ion-pairing) IPâreversedâphase LC–mass spectrometry (MS) (12). This methodology separates the oligo and its impurities, the shortmers, the parent oligo, impurities differing n+1 or n-1, and equal length impurities (n) and longmers. The shortmers and longmers are quantified by UV while the compounds in the main peak are measured by MS (see Figure 2). Diastereoisomers are formed when the phosphate backbone is thiolated. This increases separation complexity as a 19-mer length oligo will have 219 = 524288 isomers! Separations are controlled by ion-pairing reagent type, stationary phase type, gradient slope, number, and position of sulphur atoms. Methods typically have shallow gradients and low ion-pairing concentrations with tetrabutyl ammonium hydroxide being a common ionâpairing reagent.
mRNA are single strand nucleotides of 800–8000 nucleotides in length and a mass in the order of ~3000 kDa. They are synthesized using enzymes that require chromatographic purification to improve quality. From a regulatory perspective, mRNA is classed as either a biologic or gene therapy, but guidelines are vague. However, several critical quality attributes (CQAs) are typically measured including:
- Integrity
- Quantity
- Purity
- Amount of cap (uncapped form is typically inactive)
- Amount of Poly A tail (tailless form is typically inactive)
For mRNA analysis capillary electrophoretic and IP-reversed-phase LC methods were described. Örnskov said AstraZeneca has developed a charge-based separation with a low viscosity sieving media (capillary gel electrophoresis [CGE]). The gel is based on polyvinylpyrrolidone (1–2% w/w), glycerol (10–20% w/w), and 112 mM HEPES pH 7.5 (pH has to be between 6 and 9 to prevent degradation). Hydrodynamic injection is used to measure both assay and impurities and UV detection is performed at 260 nm. This methodology was good for measuring longmers, multimers, and foldmers.
The IP-reversed-phase LC method they have developed is based on a 200 Å wide pore column (100 × 2.1 mm i.d., 4-µm C4). The mobile phase is 100 mM TEA acetate–acetonitrile at low flow rate (0.2 mL/min) with a column temperature of 80 °C. It was found to be imperative to include 0.1 mM EDTA in the mobile phase to complex any metal ions in the LC system, column, or mobile phase. This improves peak shape, method robustness, and equilibrates the column more quickly. Örnskov concluded her presentation by illustrating the separation of the 858 nucleotide EPO (mRNA from Trilink) using this methodology.
The next talk was given by Adam Taylor from National Physical Laboratory (NPL) on “Metrology for Mass Spectrometry Imaging in Life Science Research” (Figure 3). NPL are working on the grand challenge Rosetta, which investigates the spatial heterogeneity of cancers. To do this, multiple mass spectrometry imaging (MSI) instrumentation is available at NPL including matrix-assisted laser desorption–ionization (MALDI), desorption electrospray ionization (DESI), secondary ion mass spectrometry (SIMS), nanoSIMS, 3D OrbiSIMS, laser ablation-inductively coupled plasma-MS (LA-ICP-MS), and rapid evaporative ionization mass spectrometry (REIMS). The volume of data produced from all these techniques is extremely large and a specialized cloud data storage facility is required for this. All data are stored in a common data format. Much research has focused on data processing, and their automated approach now allows 192 tissue samples (0.5 TB of data) to be processed in two weeks when it historically took 120 days.

The instrumental MS challenges require a balance of spatial resolution, sensitivity, speed, and data file size. NPL has developed high repetition lasers that allow extremely fast “raster” lasering ionization of tissue samples. The company has also developed different MS sources geometries, which lead to increases in ion yields, and it has developed a postâionization approach, which dramatically increases sensitivity by improving ionization of neutral species.
Significant research is also undertaken on sample preparation to improve tissue sequence coverage. Most samples are formalin-fixed paraffin-embedded (FFPE), but fresh frozen samples offer much better insight into cancer morphology; they do degrade rapidly and are also difficult to obtain during invasive cancer surgery. Liquid extraction surface analysis (LESA) is used to further decrease ion suppression by salt removal, and is often used in combination with nano-LC–MS for very small surplus.
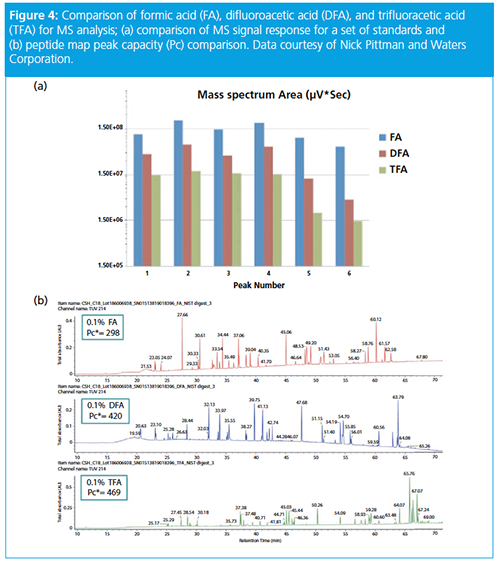
The final lecture in the session was given by Nick Pittman of Waters, who gave a presentation on “A Novel LC–MS Approach for Enhancing Protein Separations with Improved MS Sensitivity”. He opened his presentation by discussing the utility of trifluoroacetic acid (TFA) as an ion-pairing reagent for biomolecule analyses because it pairs with protonated nitrogen groups on molecules, decreasing stationary phase silanol interactions and improving peak shape. The use of formic acid (HCOOH) gives poorer retention and poorer peak shapes than TFA but much better MS response (typically 12-fold or higher sensitivity). The use of bridged ethyl hybrid phases gives better peak shape than analogous silica phases, but the charged surface hybrid technology allows a mixture of TFA and HCOOH (0.02% and 0.08% v/v, respectively) to be used, which improves MS response over TFA or HCOOH alone.
Waters has recently introduced difluoroacetic acid (DFA) as an alternative to TFA. While retention is lower than TFA, MS response was shown to be at least a twofold improvement in sensitivity (see Figure 4). In addition to this, Waters has also released a new 2.7-µm 450 Å solid core polyphenyl particle that was designed for the analysis of biomolecules. This was exemplified in the analysis of a Pfizer antibody–drug conjugate (ADC). The original method used 0.1% v/v TFA, 10% v/v IPA at 80 ºC (peak capacity of 135), but was translated to 0.15% v/v DFA, no IPA at 70 ºC (peak capacity of 151 and MS sensitivity increase of 3–4).
The lunch break provided an opportunity to interact with the vendors and discuss their products (Figure 5). The first lecture after lunch was provided by Timothy Cross from Thermo Fisher Scientific, who discussed “New Paradigms in Liquid Chromatography Separation Efficiency”. He discussed the company’s new UHPLC systems and the benefits of dual LC, LC/LCMS, and inverse gradient workflows.

The next vendor presentation was delivered by Shaun Pritchard from Agilent Technologies on “New Developments in SFC and Future Concepts”. He discussed the ability to perform very large injections of up to 80 microlitres on the company’s system, thereby addressing a critical issue with respect to hopes for increasing uptake of supercritical fluid chromatography (SFC). Pritchard also presented work from Agilent collaborators investigating LC × SFC for achiral × chiral analysis (Sabine Heinisch’s group at Institut des Sciences Analytiques, France) and LC × SFC utilizing trap columns for comprehensive sample analysis.
The final presentation in the session was delivered by Kevin Treacher of AstraZeneca on “Approaches for the Characterization and Analysis of Advanced Drug Delivery Systems”. Treacher started his presentation by discussing the enhanced permeation and retention (EPR) effect, which is believed to lead to increased permeation of pharmaceutical drugs at their designated sites of action. This leads to an increase in space between the minimum effective dose and the maximum tolerated dose. This phenomenon has led to the development of pharmaceutical nanoparticle design and their release properties are governed by many factors including size, charge, solubility, cytotoxicity, system clearance, and immune system response.
Treacher said the focus of his talk would be drug conjugates (which include formulations like drug-polymer conjugates, dendrimers, and ADCs) and nanoparticles (which include micelles, liposomes, polymersomes, inorganic, and polymeric nanoparticles). He said that to characterize these formulations he would discuss SEC, SFC, and asymmetric flow field-flow fractionation (AF4).
Nanoparticle size properties are very important. For parenteral administration they are typically sterilized by filtration so must be <200 nm, but also must be large enough to avoid excretion via the kidney (>5–10 nm). Particle characteristics, such as drug loading, particle morphology, and coating type, are also very important; the particles are often coated in polyethylene glycol (PEG) to make them transparent to the immune system. Treacher noted that they are often assembled using poly(L,Dâlactide-co-glycolide) or polycaprolactone, which degrade to benign products in vivo.
The main instrumentation approach to characterize these particles is via SEC with quaternary array detection (QDA). The detector array may include RI, UV, rightâangle light scattering (RALS), lowâangle light scattering (LALS), and differential viscometry, which allow determination of important particle properties such as concentration (RI), molecular weight (light scattering), intrinsic viscosity, hydrodynamic radius (viscometer), and solvent interaction (Mark-Houwink coefficient).
Nanoparticle (in this example dendrimers) charge characteristics are very important. Positively charged particles have been found to be cytotoxic and therefore neutral or negatively charged particles are required (13). A particle size of >80 nm is advantageous and a drug loading of 25–30% w/w. Dendrimeric constructs are “grown” based on a poly(L-Lysine) core with either a poly([2-alkyl]-2-oxazoline)s or PEG outer shell controlling the external environment of the particle. Treacher described how the AstraZeneca dendrimer will typically be 20 to 150 kDa and around 30 to 200 kDa with the drug loading. Using a SEC-quaternary detector array (QDA) system, he highlighted its potential for aggregate determination because the physicochemical properties of these systems in solution can be complex.
He also presented an example of particle size distribution determination using AF4 and dynamic light scattering (DLS) detection. He noted that by using this arrangement a shape factor (Rg/Rh) may be determined, which may be used to probe particle-protein interactions.
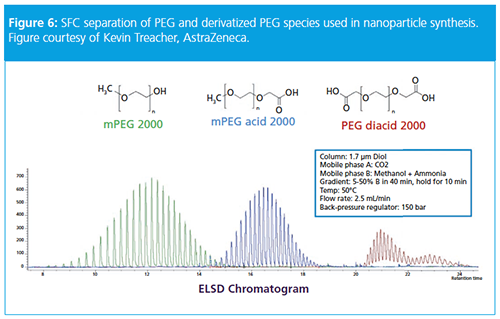
In a final example, Treacher discussed characterization of PEG and PEG-derivative excipients. He noted that a common excipient is PEG2000, which improves drug solubility. Using SFC he showed impressive separations of mPEG2000, mPEG acid2000, and PEG diacid using a methanol–ammonium hydroxide mobile phase gradient (5–50% v/v methanol) and a 1.7-µm diol column (see Figure 6). Using this methodology, it was possible to baseline resolve all the individual oligomers of each of the excipients.
After the break, the first presentation in the final session was delivered by Ben Baars of VUV Analytics on “Vacuum Ultraviolet Detection in Gas Chromatography; What are the Perspectives With This New Detector” (Figure 7). He began his presentation by noting that the vacuum ultraviolet name of the technique referred to the wavelength range of the detection (approximately 120–240 nm) rather than the technique being operated under vacuum conditions. In this UV wavelength range, almost all molecules absorb radiation and is therefore a near universal detection (including water and oxygen) (14). The only molecules transparent to the detector are helium and hydrogen, making it an excellent technique to couple to gas chromatography (GC).

In a final note, Baars mentioned that this was his final ever presentation as he is taking a well-earned retirement; The Chromatographic Society and RSC separation science group wish him well.
The last presentation of the meeting was delivered by Aditya Malker of Owlstone Medical Ltd., who discussed “Development of Technology and Workflows for Metabolite Profiling of Human Breath”. Malker started the presentation by discussing the formation of endogenous volatile organic compounds (VOCs) produced during body cell function, which typically number over 1000 in any single breath. Exogenous VOCs are also produced and are dependent on factors like food intake, exercise, medication, and smoking. He said that while breath sampling to identify disease markers is well recognized, to date fractional exhaled nitric acid (FeNO) and Helicobacter pylori breath tests are in widespread use due to difficulties in “breath bag” sampling, which suffer chemical losses over time, are vulnerable to airborne contaminants, and are difficult to store or transport.
Endogenous VOCs are good markers of bodily functions as the blood sweeps the whole body in one minute and the VOCs are expelled from the lungs during breathing. Owlstone has developed a breath sampler that learns a patient’s breathing patterns and can collect samples from different parts of the airways.
Following final questions, Sam Whitmarsh thanked the audience for their attendance and closed a very informative meeting. This edition of the meeting series highlighted the continuing developments within the field of separation science and brought the audience up to date with many emerging analytical trends. It illustrated the novelty and continuing evolution of chromatography and the clear progression of separation science from the previous two instalments of this meeting.
The Chromatographic Society’s next meeting will be the Grass Roots IV event “Introduction to Biopharmaceutical Analysis”, which will be held in Church Stretton 4–7 October 2019. Further details and registration may be found at https://chromsoc.com/events/. Future RSC separation science group meetings may be found on their website at https://www.rsc.org/Membership/Networking/InterestGroups/separationscience/
References
- G. Groeneveld et al., J. Chromatogr. A1569, 128 (2018).
- B.W.J. Pirok et al., Anal. Chem. 89, 9167 (2017).
- A. Baglai et al., Anal. Chim. Acta.1013, 87 (2018).
- B.W.J. Pirok et al., J. Sep. Sci. 41, 69 (2018).
- B. Wouters et al., J. Chromatogr. A1491, 36 (2017).
- G. Haugaard and T.D. Kroner, JACS70, 2135 (1948).
- G. Guiochon et al., J. Chromatogr.255, 415 (1983).
- E. Davydova et al., J. Chromatogr. A1271, 137 (2013).
- J.R.C Dizon et al., Additive Manu.20, 44 (2018).
- S. Sandron et al., Analyst24, 1319 (2014).
- T. Adanopoulou et al., J. Chromatogr. A1577, 120 (2018).
- S.G. Roussis et al., Rapid Comm. Mass. Spec.32, 1099 (2018).
- R.M. England et al., Polym. Chem.7, 4609 (2016).
- K.A. Schug et al., Anal. Chem.86, 8329 (2014).
Paul Ferguson is a separation science specialist at a large biopharmaceutical company in the UK. He has worked in the pharmaceutical industry since 1999 following a postdoc at Imperial College London on capillary electrochromatography (CEC) with Norman Smith. Paul has particular interests in UHPLC, SFC, CE, chiral separations, formulated drug sample preparation, green analytical chemistry, and method development. He is a past winner of the Desty Memorial lecture prize (2002), a Fellow and Chartered Chemist in the RSC, and is a visiting lecturer at King’s College London. He is currently Honorary Secretary of The Chromatographic Society. He has previously served as Vice President for the Society from 2009 to 2014 and President from 2014 to 2017. He has organized or coâorganized several successful symposia for the Society since 2007 including the inaugural Grass Roots educational event held in Grasmere in 2016.
John Lough has taught extensively on separation science, primarily across pharmaceutical sciences courses at his home university, including on the MSc Drug Discovery and Development programme (University of Sunderland) which he leads. He favours the use of industry-orientated, problem-solving laboratory classes on the university’s modern analytical instrumentation as a vehicle for giving students the skills and knowledge they need for employment in the pharmaceutical industry. In his research he has covered a wide range of LC themes and LC applications, but he is currently revisiting his longstanding interests in chiral LC and stationary phase selectivity.

Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).
Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










