Mass Spectrometry: The Premier Analytical Tool for DMPK Scientists in a Drug Discovery Environment
LCGC North America
An overview of applications of various types of MS systems in drug discovery efforts, including in vitro and in vivo screening assays.
This article focuses on current applications of various types of mass spectrometry systems to new drug discovery efforts.
Mass spectrometry (MS) has been an important analytical tool for scientists working in the drug metabolism and pharmacokinetics (DMPK) arena for several decades. Before 1990, the use of MS was restricted, in most cases, to metabolite identification studies (1–3). The early 1990s brought the commercialization of electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI). Those ionization techniques made MS an essential analytical tool for DMPK scientists (4–7). In the mid-1990s, when commercial, high performance liquid chromatography–tandem mass spectrometry (HPLC–MS-MS) systems became commonplace, HPLC–MS-MS became their premier analytical tool (4,5,8–11).
For DMPK scientists, HPLC–MS-MS is now the analytical tool of choice in all areas of research (4,8,12,13). The goal of discovery DMPK is to screen multiple compounds, assess their inherent DMPK properties, and then perform additional metabolism studies on a subset of compounds likely to proceed to drug development (14,15). This column installment focuses on current applications of various types of MS systems to new drug discovery efforts.
Types of Mass Spectrometers
Though scientists outside the field of MS may view all MS systems as more or less the same, the reality is quite different. Not only do mass spectrometers differ by type, but MS-MS systems, which are common, can combine two different types of MS systems into a single "hybrid" MS-MS system.
An extensive discussion of all these systems and how they work transcends the scope of this article. I would therefore direct readers who desire such a comprehensive review to previously published works (16–19).
Mass spectrometers can be differentiated by their mass-resolution capabilities. A low-resolution mass spectrometer can distinguish compounds that differ by at least 1 Da. Thus it can distinguish a compound detected at m/z 523 from compounds that are at least 1 Da higher (m/z 524) or 1 Da lower (m/z 522). The quadrupole (Q) MS and ion-trap (IT) MS systems are examples of low-resolution MS systems. A high-resolution mass spectrometer can measure the exact mass of a compound: That is, the mass to an accuracy of up to four decimal places (for example, 523.4267). High-resolution MS (HRMS) systems can therefore differentiate compounds that differ in mass by as little as 0.01 Da. Examples of such systems include time-of-flight (TOF) and orbital trap (Orbitrap, Thermo Fisher Scientific) systems.
MS-MS systems combine various types of mass spectrometers into a single system. A very common MS-MS system is the triple-quadrupole (QQQ) MS-MS system; that is, it uses three quadrupoles. Hybrid MS-MS systems combine two types of MS systems in one instrument. For example, a QTOF MS-MS system combines a quadrupole system and a TOF system, and a QIT MS-MS system combines a quadrupole system and a linear IT system (QTRAP, AB Sciex) (20–22). I address the utility of these various MS-MS systems below, in a discussion about how MS is used for various drug metabolism assays.
Screening Assays in Discovery Drug Metabolism
Drug metabolism scientists use multiple screening assays to understand the properties of new chemical entities (NCEs). As shown in Figure 1, the new drug discovery paradigm comprises multiple stages, which include various screening assays. It also includes more complicated studies that apply when compounds are considered as potential development candidates. The goal of the screening assays is to filter out compounds that are not suitable as potential development candidates and to identify compounds that possess good drug-like properties (23–27). The assays are subdivided into the categories in vitro and in vivo, both of which I discuss here.
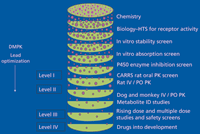
Figure 1: Stages in new drug discovery. The levels refer to the assay rules (see text). Figure was adapted with permission from reference 78.
In Vitro Screening Assays
A frequently used in vitro screening assay, the Caco-2 cell assay, is a permeability assay based on an adenocarcinoma cell line that develops in the human colon. The goal of this assay is to measure the intestinal permeability of a compound. The assay must measure the concentration of the test compound on two sides of the cell (apical and basolateral). Details of the assay appear in various publications (23,28,29). Currently, the samples are assayed using HPLC–MS-MS, as described by several authors (30–32).
A related in vitro screen is the parallel artificial membrane permeability assay (PAMPA) (33–38), which uses an artificial membrane to provide a pure permeability assay (there are no transporters in this system). Samples from these assays are typically analyzed using standard HPLC–MS-MS methods (39), although at least one report describes using a "chip-based" nano-ESI method for these samples (40).
The metabolic stability assay is another common in vitro screen in which the NCEs are added to liver microsomes or hepatocytes and incubated for up to 4 h. Aliquots taken from the incubation at specific time intervals are quenched with organic solvent (typically acetonitrile), stopping the reaction. Korfmacher and colleagues (41) described a fully automated system for assaying microsomal stability study samples using a single-quadrupole HPLC–MS system. Recently, most researchers have assayed these samples by various HPLC–MS-MS methods (42–47). The primary goal of these studies is to differentiate the NCEs that are metabolically stable from those that are unstable. Calculating the in vitro clearance (CLin) for the compounds is the secondary goal. The CLin can then be used to predict the in vivo clearance (CL) for the compounds by using various scaling factors (48–54).
The cytochrome P450 (CYP) inhibition assay is another common in vitro screen (14). These studies are important because of the potential for drug–drug interactions when dosing compounds whose enzyme-inhibition activity is significant (55–57). The many human CYP isoforms have brought about a steady evolution of the CYP inhibition assay. Thus, initial methods reported for CYP 2D6 or 3A4 (58–60) were followed by methods for both of these isozymes in one assay (61). Soon, single assays for multiple CYPs emerged (62): For example, Plumb and colleagues (63) describe a fast ultrahigh-pressure liquid chromatography (UHPLC)–MS-MS assay for six CYP substrates.
Finally, protein binding is another in vitro screening assay in widespread use. The protein binding assay measures the percent free (versus percent bound) levels of an NCE in animal or human plasma (23). The assay is typically performed using ultrafiltration or equilibrium dialysis to separate the free fraction from the bound fraction. The assay for the free fraction must be sensitive enough to measure free fractions of 0.1% for compounds that are highly bound to plasma proteins. The need for a sensitive assay means that the analytical method of choice for protein binding studies is HPLC–MS-MS (64–69).
In Vivo Screening Assays and PK Studies
The primary in vivo screening assay is the pharmacokinetics (PK) screen typically carried out in rats or mice. The goal of this screen is to obtain an initial picture of the PK properties of the NCEs tested. At a large pharmaceutical company, the need can arise to test 50–100 compounds/week in this screen. The simplest PK screen uses one dose route, typically oral or intravenous, for all the compounds. The excellent sensitivity of HPLC–MS-MS confers the ability to perform a complete PK study, with 6–9 time points, using only one rat. Normally, one or two replicates are desired for a PK study, so a PK screen can be performed on one compound with one dose route by using two or three rats.
The practice of cassette dosing reduces the number of samples assayed as well as the number of rats needed. In a typical cassette-dosing study, 5–9 compounds are mixed and dosed into one rat (70–72). This process was popular for many years, but it has recently fallen out of favor (73–75) because drug–drug interactions can, in some cases, lead to incorrect PK parameters (76).
Cassette-accelerated rapid rat screen (CARRS), an alternative PK screening procedure, has also been used for many years (77). It is a systematic method for oral PK screening in rats. In the CARRS method, each compound is orally dosed into two rats, and then blood from each is sampled at six time points. The samples are pooled across the two rats, giving six total plasma samples to assay for each compound. For assay purposes, the compounds were grouped into cassettes of six, and a minimal standard curve was used for each compound. Thus, all samples and standards for the six compounds in one cassette could fit onto one 96-well plate (78).
The CARRS assay was used successfully for more than 5000 compounds (79). Liu and colleagues describe a similar PK screen, called "snapshot PK," that they developed for mice (80). In both of these PK screening assays, the samples were assayed using HPLC–MS-MS systems (77,78,80).

Table I: Rules for quantitative assays at various levels in drug discovery and development*
One way to be more efficient in the discovery bioanalytical arena is to define various levels for the different types of assays. As discussed by Korfmacher (78), it makes sense to use a simpler assay for an early screen, where only a few samples or compounds are assayed, and then use a more robust assay for PK studies (see Figure 1). As shown in Table I, this concept has been formalized into a set of rules for performing bioanalytical studies at the various stages of new drug discovery (78). In this paradigm, screening studies can be assayed using a two-point standard curve, though PK studies in dogs or monkeys would require multipoint standard curves. It is nonetheless important to follow a standardized process for producing the standard curves and assaying the samples from the PK studies. As shown in Figure 2, you can perform these tasks by adopting a systematic procedure for preparing the standards and samples, running the assay, and then issuing the results to the discovery team. To perform these steps in a time-appropriate way, you must be able to develop the HPLC–MS-MS assay quickly. Xu and colleagues (81) described a procedure for quickly developing HPLC–MS-MS assays in a drug discovery setting.

Figure 2: Schematic showing the various steps taken when assaying compounds in a discovery PK setting. Figure was adapted with permission from reference 78.
Matrix Effects
A significant factor to understand when using MS for assaying samples from PK studies is the potential for problems arising from matrix effects. As described by Mei (82), matrix effects can be caused by various components in the sample or, in some cases, by the sample preparation method (83). In HPLC–MS-MS assays, they can be defined simply as coeluted components that result in ion suppression or ion enhancement (82). In either case, matrix effects can lead to incorrect results. Typically, components of the plasma matrix cause matrix effects in PK samples. In one important manuscript, King and colleagues (84) show a process for visualizing the extent of matrix effects in an assay. Matuszewski and colleagues (85–87) describe useful procedures for measuring the matrix effects in an assay. It is important to evaluate any bioanalytical method for matrix effects, as part of the method development strategy (81).
Mass Spectrometry Imaging
Mass spectrometry imaging (MSI) is an additional way to use MS in DMPK studies. A relatively recent technique, MSI was initially developed for protein and peptide analysis, and the technique relied on matrix-assisted laser desorption–ionization (MALDI) connected to a TOF-MS system. Subsequently, its use was extended to NCE studies (88–90), requiring small-molecule analysis. A switch to MALDI on a QTOF MS-MS system became necessary because the specificity of MS-MS was necessary to distinguish the small molecules from the background ions the matrix produced (90).
In a typical experiment, tissue from a laboratory animal dosed with an NCE is frozen. Then a thin slice (typically 10 μm) is attached to a MALDI plate. Finally, the matrix is applied to the sample, which undergoes assay (88). The result is a "picture" showing where the NCE is located on the tissue sample. This technique can also locate major metabolites on the sample (91–93). More recently, MSI has provided analyte imaging for whole-mouse and whole-rat sections, providing results similar to those obtained by whole-body autoradiography analysis (94–96).
A new ionization technique, desorption electrospray ionization (DESI), has also been used for MSI studies. DESI does not require a matrix for the assay, a considerable advantage (97). Another new MS technique, liquid extraction surface analysis mass spectrometry (LESA-MS), has also been evaluated as a tool for tissue-profiling analysis (98). Like DESI, LESA-MS does not require a matrix.
Metabolism Studies: Metabolite Identification
The two types of metabolism studies are in vitro and in vivo. The most frequently used in vitro metabolism study involves incubating the NCE in liver microsomes or hepatocytes (23). Though performable at various substrate concentrations, the results of in vitro studies are often concentration-dependent. Typical concentrations for a test compound are 1–10 μM. If the goal of the study is to be physiologically relevant, then a test concentration of 1 μM would be reasonable (23). Previously, performing metabolite identification studies on microsomal incubation studies that used 1 μM concentrations for the test compounds was difficult. However, given current MS technology, doing so is no longer a challenge. Typically, in vivo studies are based on dosing a compound to a laboratory animal, collecting bile and urine from the animal for 1–2 days, and then assaying the bile or urine to find metabolites of the dosed compound.
Various types of MS systems have been used for metabolite identification studies over the last 20 years. For much of that time, the most effective MS tool was the triple-quadrupole MS-MS system (5,99,100). The advantage of this system lies in its ability to use neutral-loss scanning as well as precursor-ion scanning as tools to search for metabolites. When metabolites were found, the product-ion scanning mode was used to obtain data for their structural elucidation (7,99).
The next MS tool used for metabolite identification studies was the IT-MS system. Extremely useful for structural analysis, IT provided researchers the ability to perform MSn assays (101,102). The third MS tool for metabolite identification studies is the QTOF-MS-MS system, which can provide HRMS data in MS or MS-MS mode (102–104).
For many years, the best way to perform metabolite identification studies was to use all three MS-MS systems — triple quadrupole, IT-, and QTOF-MS — to find the metabolites, and then perform the structural elucidation studies (102,105). With the development of new software tools like mass-defect filtering (explained below), the importance of the QTOF-MS-MS system as a tool for metabolite identification has increased dramatically (103,104).
An interesting development was the QIT-MS system, a hybrid system that combined the capabilities of a triple-quadrupole system with an IT system. Such a combination proved useful for both quantitative analysis and metabolite identification studies (20–22). The QIT-MS system provided DMPK scientists with the ability to perform quantitative analysis and metabolite identification on the same samples in a single analysis (106,107,108).
Perhaps the most noteworthy new MS system in recent years is the orbital trap MS system (109), an HRMS system that can provide mass resolution from 25,000 to 100,000. This system, now available in several configurations, has proven to be another excellent tool for metabolite identification studies (19,110–117).
The MIST Impact
Metabolite identification has always been an important part of the drug discovery paradigm. It has gained additional importance recently because of updated guidelines for metabolites in safety testing (MIST) issued by the U.S. Food and Drug Administration (FDA). A plethora of studies describe how MS tools can ensure that the evaluation of NCEs are consistent with the revised MIST rules (118–123). Because of the MIST guidelines, DMPK scientists have been seeking ways to find human metabolites during multiple-dose Phase 1 clinical studies. Ma and Chowdhury (124) recently published an article describing how HRMS can, when used with various software tools, find metabolites in samples from clinical studies.
Another important area for metabolite identification is reactive metabolite screening. A common procedure looks for glutathione (GSH) adducts of the reactive metabolites from various in vitro or in vivo studies (111,125–131). In one of the more interesting techniques, Mutlib and colleagues (131) describe the utility of mixing (1:1) unlabeled GSH with GSH labeled with glycine-13 C2,15 N, thus providing a unique isotope pattern to find GSH metabolites. The metabolites would show an unusual ratio of 1:1 for the monoisotopic [M+H]+ ion and a second ion that was 3 Da higher.
The Future of Mass Spectrometry in DMPK Studies
The future of MS and DMPK studies is clear in one sense: MS will continue to be the premier analytical tool for both qualitative and quantitative (qual/quan) DMPK assays. What is changing is that HRMS is becoming the main tool for metabolite identification studies and for quantitative assays, as well. It is entirely predictable that HRMS will become the primary tool for quantitative applications of drug discovery in the future (108,132).
The ability to perform qual/quan experiments is a main driver for the change to using HRMS for quantitative applications of discovery, for both in vitro and in vivo applications (108,132). In simple terms, qual/quan assays derive data from the analysis of one sample that you can use for both qualitative and quantitative analysis of the sample. For example, you can use a full-scan (m/z 100–1000) assay of a metabolite-stability sample on an HRMS system to perform a quantitative analysis of the test compound, and additionally use those data to look for metabolites of the test compound.
The regulated bioanalytical field will require more time to convert from HPLC–MS-MS assays on a triple-quadrupole system to HPLC–HRMS on a QTOF system. The other change that will come to the quantitative assay arena is the ability to assay small sample volumes. One driver for this change is the current zeal to use dried blood spots as samples in clinical and preclinical studies (133–135). Admittedly, only a few pioneering reports of discovery PK assays based on small volumes of blood or plasma have come to light. Nevertheless, I expect we will soon be routinely handling small volumes (10 μL or less) of blood or plasma (136–138). My final prediction is that MSI will become a commonplace tool for drug-discovery, DMPK, in vivo studies. Moreover, it will prove very helpful in providing an early understanding of the distribution of NCEs and their major metabolites.
References
(1) W.A. Korfmacher et al., J. Anal. Toxicol. 11(4), 182–184 (1987).
(2) R.J. Perchalski, M.S. Lee, and R.A. Yost, J. Clin. Pharmacol. 26(6), 435–442 (1986).
(3) L.D. Betowski et al., Biomed. Environ. Mass Spectrom. 14(12), 705–709 (1987).
(4) Using Mass Spectrometry for Drug Metabolism Studies, W. Korfmacher, Ed. (CRC Press, Boca Raton, Florida, 2005).
(5) W.A. Korfmacher et al., Drug Discov. Today 2, 532–537 (1997).
(6) Integrated Strategies for Drug Discovery using Mass Spectrometry, M.S. Lee, Ed. (John Wiley & Sons, Inc., Hoboken, New Jersey, 2005).
(7) M.S. Lee et al., Mass Spectrom. Rev. 18(3–4), 187–279 (1999).
(8) W.A. Korfmacher, Drug Discov. Today 10(20), 1357–1367 (2005).
(9) M. Jemal et al., Rapid Commun. Mass Spectrom. 12(19), 1389–1399 (1998).
(10) M. Jemal, Biomed. Chromatogr. 14(6), 422–429 (2000).
(11) D.T. Rossi and M. Sinz, Mass Spectrometry in Drug Discovery (Marcel Dekker, Inc., New York, New York, 2002).
(12) Y. Hsieh, Expert Opin. Drug Metab. Toxicol. 4(1), 93–101 (2008).
(13) B. Ackermann et al., Annu. Rev. Anal. Chem. 1, 357–396 (2008).
(14) R.J. Riley et al., Curr. Drug Metab. 3(5), 527–550 (2002).
(15) W.A. Korfmacher, Mini Rev. Med. Chem. 9(6), 703–716 (2009).
(16) G. Hopfgartner, in Mass Spectrometry in Drug Metabolism and Disposition: Basic Principles and Applications, M. Lee and M. Zhu, Eds. (John Wiley & Sons, Hoboken, New Jersey, 2011), pp. 257–290.
(17) Using Mass Spectrometry for Drug Metabolism Studies: Second Edition, W.A. Korfmacher, Ed. (CRC Press, Boca Raton, Florida, 2009).
(18) R.K. Boyd et al., Trace Quantitative Analysis by Mass Spectrometry (John Wiley & Sons, Hoboken, New Jersey, 2008).
(19) R. Ramanathan, Mass Spectrometry in Drug Metabolism and Pharmacokinetics (John Wiley & Sons, Hoboken, New Jersey, 2009).
(20) G. Hopfgartner et al., J. Mass Spectrom. 39(8), 845–855 (2004).
(21) G. Hopfgartner et al., in Using Mass Spectrometry for Drug Metabolism Studies, W.A. Korfmacher, Ed. (CRC Press, Boca Raton, Florida, 2005) pp. 277–304.
(22) R. King et al., Curr. Drug Metab. 7(5), 541–545 (2006).
(23) E. Kerns and L. Di, Drug-like Properties: Concepts, Structure Design and Methods from ADME to Toxicity Optimization (Elsevier/Academic Press, New York, New York, 2008).
(24) L. Di and E.H. Kerns, Curr. Opin. Chem. Biol. 7(3), 402–408 (2003).
(25) L. Di and E.H. Kerns, Curr. Opin. Drug Discov. Devel. 8(4), 495–504 (2005).
(26) L. Di et al., Curr. Pharm. Des. 15(19), 2184–2194 (2009).
(27) C.A. Lipinski, J. Pharmacol. Toxicol. Methods 44(1), 235–249 (2000).
(28) P. Shah et al., Biotechnol. Prog. 22(1), 186–198 (2006).
(29) I. Chu, in Using Mass Spectrometry for Drug Metabolism Studies, W.A. Korfmacher, Ed. (CRC Press, Boca Raton, Florida, 2010), pp. 99–126.
(30) H.Z. Bu et al., Rapid Commun. Mass Spectrom. 14(6), 523–528 (2000).
(31) E.N. Fung et al., Rapid Commun. Mass Spectrom. 17(18), 2147–2152 (2003).
(32) K.S. Hakala et al., Anal. Chem. 75(21), 5969–5977 (2003).
(33) A. Avdeef, Expert Opin. Drug Metab. Toxicol. 1(2), 325–342 (2005).
(34) A. Avdeef et al., Eur. J. Pharm. Sci. 28(1–2), 43–50 (2006).
(35) M. Bermejo et al., Eur. J. Pharm. Sci. 21(4), 429–441 (2004).
(36) L. Di et al., Eur. J. Med. Chem. 38(3), 223–232 (2003).
(37) C. Li et al., Biochem. Pharmacol. 75(5), 1186–1197 (2008).
(38) M. Kansy et al., J. Med. Chem. 41(7), 1007–1010 (1998).
(39) J. Mensch et al., J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 847(2), 182–187 (2007).
(40) P.V. Balimane et al., J. Pharm. Biomed. Anal. 39(1–2), 8–16 (2005).
(41) W.A. Korfmacher et al., Rapid Commun. Mass Spectrom. 13(10), 901–907 (1999).
(42) G.W. Caldwell et al., Comb. Chem. High Throughput Screen 2(1), 39–51 (1999).
(43) L.E. Chovan et al., Rapid Commun. Mass Spectrom. 18(24), 3105–3112 (2004).
(44) L. Di et al., J. Biomol. Screen 8(4), 453–462 (2003).
(45) L. Di et al., Comb. Chem. High Throughput Screen 11(6), 469–476 (2008).
(46) M. Fonsi et al., J. Biomol. Screen 13(9), 862–869 (2008).
(47) K. Kieltyka et al., Rapid Commun. Mass Spectrom. 23(11), 1579–1591 (2009).
(48) P. Baranczewski et al., Pharmacol. Rep. 58(4), 453–472 (2006).
(49) Z.E. Barter et al., Curr. Drug Metab. 8(1), 33–45 (2007).
(50) E.M. Howgate et al., Xenobiotica 36(6), 473–497 (2006).
(51) S. Inoue et al., Xenobiotica 36(6), 499–513 (2006).
(52) R.S. Obach, Drug Metab. Dispos. 27(11), 1350–1359 (1999).
(53) R.S. Obach, Curr. Opin. Drug Discov. Devel. 4(1), 36–44 (2001).
(54) M.R. Shiran et al., Xenobiotica 36(7), 567–580 (2006).
(55) M. Hutzler et al., Curr. Opin. Drug Discov. Devel. 8(1), 51–58 (2005).
(56) C. Lu et al., Drug Metab. Dispos. 35(1), 79–85 (2007).
(57) K.A. Youdim et al., J. Clin. Pharmacol. 65(5), 680–692 (2008).
(58) H.Z. Bu et al., J. Chromatogr. B Biomed. Sci. Appl. 753(2), 321–326 (2001).
(59) A.A. Nomeir et al., Drug Metab. Dispos. 29(5), 748–753 (2001).
(60) I. Kariv et al., Biomol. Screen 6(2), 91–99 (2001).
(61) I. Chu et al., Rapid Commun. Mass Spectrom. 14(4), 207–214 (2000).
(62) H.Z. Bu et al., Rapid Commun. Mass Spectrom. 15(10), 741–748 (2001).
(63) R.S. Plumb et al., Rapid Commun. Mass Spectrom. 22(14), 2139–2152 (2008).
(64) M.J. Banker et al., J. Pharm. Sci. 92(5), 967–974 (2003).
(65) I. Chu et al., Curr. Drug Metab. 7(5), 467–477 (2006).
(66) E.N. Fung et al., J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 795(2), 187–194 (2003).
(67) E.H. Kerns et al., J. Pharm. Biomed. Anal. 20(1–2), 115–128 (1999).
(68) H. Wan et al., J. Chromatogr. A 1102(1–2), 125–134 (2006).
(69) J. Zhang et al., Rapid Commun. Mass Spectrom. 24(24), 3593–3601 (2010).
(70) B.L. Ackermann et al., Curr. Top. Med. Chem. 2(1), 53–66 (2002).
(71) K. He et al., J. Pharm. Sci. 97(7), 2568–2580 (2008).
(72) T.V. Olah et al., Rapid Commun. Mass Spectrom. 11(1), 17–23 (1997).
(73) B.L. Ackermann, J. Amer. Soc. Mass Spectrom. 15(9), 1374–1377 (2004).
(74) D.D. Christ, Drug Metab. Dispos. 29(7), 935 (2001).
(75) P. Manitpisitkul et al., Drug Discov. Today 9(15), 652–658 (2004).
(76) R.E. White et al., Drug Metab. Dispos. 29(7), 957–966 (2001).
(77) W.A. Korfmacher et al., Rapid Commun. Mass Spectrom. 15(5), 335–340 (2001).
(78) W. Korfmacher, in Using Mass Spectrometry for Drug Metabolism Studies, W.A. Korfmacher, Ed. (CRC Press, Boca Raton, Florida, 2005), pp. 1–34.
(79) H. Mei et al., AAPS J. 8(3), E493–500 (2006).
(80) B. Liu et al., Drug Discov. Today 13(7–8), 360–367 (2008).
(81) X. Xu et al., Anal. Chem. 77(October 1), 389A–394A (2005).
(82) H. Mei, in Using Mass Spectrometry for Drug Metabolism Studies, W.A. Korfmacher, Ed. (CRC Press, Boca Raton, Florida, 2005), pp. 103–150.
(83) H. Mei et al., Rapid Commun. Mass Spectrom. 17(1), 97–103 (2003).
(84) R. King et al., J. Am. Soc. Mass Spectrom. 11(11), 942–950 (2000).
(85) B.K. Matuszewski, J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 830(2), 293–300 (2006).
(86) B.K. Matuszewski et al., Anal. Chem. 70(5), 882–889 (1998).
(87) B.K. Matuszewski et al., Anal. Chem. 75(13), 3019–3030 (2003).
(88) M.L. Reyzer et al., in Using Mass Spectrometry for Drug Metabolism Studies, W.A. Korfmacher, Ed. (CRC Press, Boca Raton, Florida, 2005), pp. 305–324.
(89) M.L. Reyzer et al., J. Proteome Res. 4(4), 1138–1142 (2005).
(90) M.L. Reyzer et al., J. Mass Spectrom. 38(10), 1081–1092 (2003).
(91) Y. Hsieh et al., Rapid Commun. Mass Spectrom. 20(6), 965–972 (2006).
(92) Y. Hsieh et al., J. Pharmacol. Toxicol. Methods 55(2), 193–200 (2007).
(93) Y. Hsieh et al., Methods Mol, Biol, 656, 147–158 (2010).
(94) F. Li, in Using Mass Spectrometry for Drug Metabolism Studies, Second Edition. W.A. Korfmacher, Ed. (CRC Press, Boca Raton, Florida, 2010), pp. 333–353.
(95) S. Khatib-Shahidi et al., Anal. Chem. 78(18), 6448–6456 (2006).
(96) E.G. Solon et al., AAPS J. 12(1), 11–26 (2010).
(97) J.M. Wiseman et al., Proc. Natl. Acad. Sci. U.S.A. 105(47), 18120–18125 (2008).
(98) D. Eikel et al., Rapid Commun. Mass Spectrom. 25(23), 3587–3596 (2011).
(99) W.A. Korfmacher et al., Biomed. Environ. Mass Spectrom. 19(3), 191–201 (1990).
(100) W.A. Korfmacher et al., Biomed. Environ. Mass Spectrom. 15(9), 501–508 (1988).
(101) H.K. Lim et al., J. Chromatogr. A 831(2), 227–241 (1999).
(102) K. Cox, in Using Mass Spectrometry for Drug Metabolism Studies, W.A. Korfmacher, Ed., (CRC Press, Boca Raton, Florida, 2005), pp. 229–252.
(103) R.J. Mortishire-Smith et al., Rapid Commun. Mass Spectrom. 19(18), 2659–2670 (2005).
(104) P.R. Tiller et al., Rapid Commun. Mass Spectrom. 22(7), 1053–1061 (2008).
(105) N.J. Clarke et al., Anal. Chem. 73(15), 430A–439A (2001).
(106) A.C. Li et al., Rapid Commun. Mass Spectrom. 19(14), 1943–1950 (2005).
(107) W.Z. Shou et al., J. Mass Spectrom. 40(10), 1347–1356 (2005).
(108) W.A. Korfmacher, Bioanalysis 3(11), 1169–1171 (2011).
(109) R.H. Perry et al., Mass Spectrom. Rev. 27(6), 661–699 (2008).
(110) A.C. Li et al., Rapid Commun. Mass Spectrom. 23(18), 3003–3012 (2009).
(111) S. Ma et al., Chem. Biol. Interact. 179(1), 25–37 (2009).
(112) Q. Ruan et al., J. Mass Spectrom. 43(2), 251–261 (2008).
(113) R. Ramanathan et al., in Using Mass Spectrometry for Drug Metabolism Studies, Second Edition, W.A. Korfmacher, Ed. (CRC Press, Boca Raton, Florida, 2009), pp. 205–228.
(114) H.K. Lim et al., Rapid Commun. Mass Spectrom. 21(12), 1821–1832 (2007).
(115) H. Zhang et al., J. Mass Spectrom. 44(7), 999–1016 (2009).
(116) H. Zhang et al., Rapid Commun. Mass Spectrom. 22(13), 2082–2088 (2008).
(117) M. Zhu et al., Drug Metab. Dispos. 34(10), 1722–1733 (2006).
(118) D.A. Smith et al., Bioanalysis 2(7), 1223–1233 (2010).
(119) C.B. Frederick and R.S. Obach, Clin. Pharmacol. Ther. 87(3), 345–350 (2010).
(120) D.A. Smith et al., Chem. Biol. Interact. 179(1), 60–67 (2009).
(121) D.A. Smith et al., Chem. Res. Toxicol. 22(2), 267–279 (2009).
(122) L. Leclercq et al., Chem. Res. Toxicol. 22(2), 280–293 (2009).
(123) F.P. Guengerich, Chem. Res. Toxicol. 22(2), 237–238 (2009).
(124) S. Ma et al., Anal. Chem. 83(13), 5028–5036 (2011).
(125) Z. Yan et al., Anal. Chem. 76(23), 6835–6847 (2004).
(126) G. Hopfgartner, in Using Mass Spectrometry for Drug Metabolism Studies, Second Edition, W.A. Korfmacher, Ed. (CRC Press, Boca Raton, Florida, 2010), pp. 205–228.
(127) J. Castro-Perez et al., Rapid Commun. Mass Spectrom. 19(6), 798–804 (2005).
(128) M.K. Mahajan et al., Rapid Commun. Mass Spectrom. 22(7), 1032–1040 (2008).
(129) J. Zheng et al., Chem. Res. Toxicol. 20(5), 757–766 (2007).
(130) M. Zhu et al., Anal. Chem. 79(21), 8333–8341 (2007).
(131) A. Mutlib et al., Rapid Commun. Mass Spectrom. 19(23), 3482–3492 (2005).
(132) R. Ramanathan et al., J. Mass Spectrom. 46(6), 595–601 (2011).
(133) M. Barfield et al., J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 870(1), 32–37 (2008).
(134) P. Beaudette et al., J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 809(1), 153–158 (2004).
(135) N. Spooner et al., Anal. Chem. 81(4), 1557–1563 (2009).
(136) X. Xu et al., Rapid Commun. Mass Spectrom. 19(15), 2131–2136 (2005).
(137) J. Chen et al., Anal. Chem. 78(4), 1212–1217 (2006).
(138) B.A. Ingelse et al., Rapid Commun. Mass Spectrom. 22(6), 834–840 (2008).
Walter Korfmacher is a Senior Director for Bioanalytical at Medpace Bioanalytical Laboratories in Cincinnati, Ohio. He is a leader in the field of developing strategies for the application of new MS techniques for drug metabolism participation in new drug discovery as well as using MS for tissue imaging. He has edited two books on MS and drug metabolism and has over 150 publications in the scientific literature. Direct correspondence to: w.korfmacher@medpace.com.

Walter Korfmacher
Kate Yu "MS — The Practical Art" Editor Kate Yu joined Waters in Milford, Massachusetts, in 1998. She has a wealth of experience in applying LC–MS technologies to application fields such as metabolite identification, metabolomics, quantitative bioanalysis, natural products, and environmental applications. Direct correspondence about this column to lcgcedit@lcgcmag.com

Kate Yu


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














