Mass Spectrometry in Analytical Lipidomics
Special Issues
An overview on the present state of analytical lipidomics is presented from the perspective of mass spectrometry (MS) and the MS strategies most frequently used in lipidomics are highlighted.
Lipidomics is a branch of metabolomics that is based on the analysis of all the lipids present in a biological system known as a lipidome. In recent years, this emergent field has grown sharply. This growth is mainly due to the progress that has been made in mass spectrometry (MS), the driving force behind the development of analytical lipidomics today. In particular, advances in soft ionization MS, such as electrospray ionization and matrix-assisted laser desorption–ionization, have enabled rapid and sensitive detection of a vast number of individual lipid molecular species from a single biological sample. In this article, we provide an overview from the perspective of MS on the present state of analytical lipidomics and highlight the MS strategies most frequently used in lipidomics. The benefits and shortcomings of MS both in the presence and absence of chromatographic separation techniques are assessed. Pharmaceuticals, medicine, and nutrition are some of the areas that can benefit from the study of lipidomics.
Despite the fact that lipid analysis was one of the first applications of the mass spectrograph after the ground-breaking studies by J.J. Thompson and F.W. Aston, for many years routine analysis of lipids was carried out primarily using methods other than mass spectrometry (MS), and the analysis was hindered by lipids' tendency to fragment during the analysis process (1). Nevertheless, this problem has largely been overcome because MS is increasingly used for the analysis of complex lipid mixtures (2), and in particular, for lipidomics studies. The fast development of MS instruments in recent years, specifically in soft ionization MS such as electrospray ionization (ESI) MS and matrix assisted laser desorption-ionization (MALDI) MS, has played an essential role in the characterization, identification, and quantification of a variety of lipid species from biological samples (3–6). In fact, MS analysis of lipids is frequently generalized in literature as lipidomics analysis.
Lipidomics is defined as the full characterization of lipid molecular species and their biological roles with respect to the expression of proteins involved in lipid metabolism and function, including gene regulation (7). Under this definition, lipidomics covers everything related to the study of the lipidome. As such, lipidomics covers the quantitative determination of lipids in space and time, and the study of lipid-metabolizing enzymes and lipid transporters, which determine the local concentration of lipids, their genes, and regulation. Lipidomics also is concerned with lipid function, especially, lipid–lipid and lipid–protein interactions (8). In view of all this, and from a chemical point of view, the new field of analytical lipidomics must focus on the efficient analysis of the entire profile of lipids in a biological system, known as a lipidome. Thus, a typical lipidomics experiment involves both extraction of the lipids from the biological sample (tissues, cells, and fluids) and their analysis, mainly by MS platforms with or without previous chromatographic separation (Figure 1). Although lipidomics analysis often uses crude lipid extracts, the extraction of lipids from samples is a complex task in lipidomics approaches and in lipid analysis in general (9). In this review, however, we focus on the analysis of the lipidome by MS, in what is regarded as, MS analytical lipidomics.
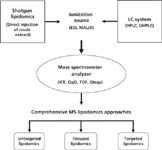
Figure 1: Comprehensive MS lipidomics approaches.
Many modern technologies, including MS, nuclear magnetic resonance (NMR), fluorescence spectroscopy, and microfluidic devices have been developed to identify, quantify, and understand the structure and function of key metabolic nodes in lipidomics. In the development of lipidomic platforms, MS techniques lead the field in the characterization, identification, and quantification of a vast variety of lipids. MS, particularly using ESI as an interface, is the principal enabling technology for tackling the lipidome (10–13). There is also increasing evidence that MALDI-MS is very useful in lipidomics research (14). The complexity of the samples under investigation evidently benefit from the use of high resolution, accurate mass and tandem MS equipment, and this is why Fourrier-transform ion cyclotron resonance mass spectrometry (FT-ICR-MS), orbital trap and quadrupole time-of-flight mass spectrometry (QTOF-MS) systems are so often cited in the literature on lipidomics (15–17). Triple-quadrupole instruments (QqQ) (5,18) also have proved to be valuable in class-specific detection through precursor ion and neutral-loss scanning MS approaches.
MS lipidomics analysis can be performed either using crude extracts directly or coupled with a chromatographic system (normally a liquid chromatography [LC] system). When direct injections of organic extracts are used, the technique is known as shotgun lipidomics analysis (3). A number of researchers claim that chromatographic separation is no longer required and can be replaced by exploiting the unique chemistries of different lipid classes, even extremely minor ones. This occurs when multidimensional MS-based shotgun lipidomics (MDMS-SL) is performed (19). In this case, intrasource separation takes the place of the chromatographic system. Nevertheless, problems arising from ion suppression in the source, mass spectra complexity, or the common presence of lipid isomers justify the combined use of chromatography and MS.
In addition, several practical MS strategies or approaches have been developed to determine the inherent complexity of each lipidome, which vary according to the particular information that the researcher is seeking. These comprehensive MS analyses are known as global or untargeted lipidomics, focused lipidomics, and targeted lipidomics and will be discussed in this review in relation to the MS platform.
Ionization MS Techniques in Analytical Lipidomics
Currently, ESI and MALDI MS are the most commonly used MS platforms for lipidomic studies, and of these two the former is the most popular. Both techniques are considered "soft" ionization MS techniques, and their wide use in analytical lipidomics methodologies is due to the fact that the initial molecules do not fragment when the ions are formed. The importance of these techniques was publicly recognized when the inventors of ESI and MALDI (Fenn and colleagues [20] and Tanaka and colleagues [21], respectively) shared the Nobel Prize for Chemistry in 2002 (22).
ESI-MS was initially developed by Fenn and colleagues for the analysis of biomolecules (20). This technique is based on the formation of gaseous ions from polar, thermally labile, and mostly nonvolatile molecules. It is therefore very suitable for polar lipids, such as phospholipids. Currently, the most widely used mass spectrometers with electrospray ion sources are QqQ, quadrupole ion trap (Q-IT), ion trap (IT), and QTOF instruments. ESI is also coupled to FT-ICR mass analyzers. They all differ in their mass accuracy (the error in the exact determination of mass compared to the theoretical value) and resolution (the value of mass m divided by the mass difference Δm between two ion profiles with a small mass difference). The "soft" ionization process associated with ESI-MS results in decreased molecular ion decomposition, better reproducibility, and lower detection limits compared to fast atom bombardment mass spectrometry (FAB-MS), the first analytical platform used to analyze intact lipids (23).
MALDI-MS is based on the use of a "matrix" that initially absorbs the energy from the laser and mediates the generation of ions. When the pulsed laser beam hits the sample, normally cocrystals of the matrix and the analyte, its energy is absorbed primarily by the matrix, which occurs in far greater quantities than the analyte. As a result, the matrix is vaporized, carrying intact analyte molecules into the vapor phase. During the process of expansion of this gas cloud, ions are exchanged between the matrix and the analyte, leading to the formation of charged analyte molecules. MALDI is often, but not exclusively, coupled with a TOF mass analyzer. It is also coupled with FT-ICR mass analyzers. Although ESI is the most popular ionization MS technique in the development of analytical lipidomics methodologies, there is a continuously increasing interest in MALDI-MS in relation to its applications in lipid analysis (14,24). Nevertheless special attention must be paid to quantitative aspects of MALDI MS because they are normally considered to be the "weak" point of the method, particularly if complex lipid mixtures are being analyzed.
Comprehensive MS Approaches in Analytical Lipidomics
Cellular lipidomes are highly complex and variable. Tens of thousands of possible lipid molecular species are likely to be present in the cellular lipidome at attomole to nanomole concentrations of lipids per milligram of protein (25). In response to the complex nature of the analysis, a series of comprehensive MS approaches have been developed, namely untargeted lipidomics, focused lipidomics, and targeted lipidomics (Figure 1). They are discussed below.
Untargeted lipidomics develops MS methods to detect all lipids (or a certain type of lipid) contained in extracted lipid samples without preliminary information on the molecular ions and their fragmentations. The strategy behind these methods is to subject all detected peaks to further analysis by using computational sorting (26) or multivariate pattern-recognition analysis (27,28) to group the ions. High-resolution FT-MS provides exceptional resolution and accuracy for use in untargeted lipidomics (26,29). Q-IT (27) and QTOF (30) mass spectrometers are also effective for this kind of global lipidomics analysis. The direct profiling of total lipid extracts can be achieved on a hybrid mass spectrometer using high-resolution survey spectra as a rapid screening tool (28); lipids were identified by accurately determined masses. The use of new matrixes for MALDI-MS (for example, 2,4,6-trihydroxyacetophenone) offers the possibility of less complex mass spectra (31). Prior separation of the lipids in LC systems enables the accurate identification of the individual molecular species (30). The untargeted lipidomics strategy provides the greatest number of individual compounds that can be analyzed in a single experiment and has a great capacity for finding unexpected molecules. However, it is the least sensitive of the three lipidomics strategies because of the complex spectra obtained, which are characterized by significant baseline noise.
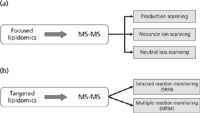
Figure 2: Focused and targeted lipidomics approaches.
The focused lipidomics strategy is used for detecting molecules in certain categories while using comprehensively specific fragments (product and precursor ion scanning) or neutral loss caused by a specific feature of the partial structures of the molecules (neutral-loss scanning). Figure 2 shows these variations of tandem MS (MS-MS). Product-ion scanning is applied to detect the fragments from an ion of interest. Precursor-ion scanning analyzes lipids from the same class or subclass. Neutral-loss scanning is used to analyze lipids that lose the same fragment on collision. Table I shows the diagnostic ions used to identify the main lipid classes in precursor-ion or neutral-loss scanning experiments. Methods applying this strategy are used for the comprehensive analysis of lipids with structural similarities. The important aspect of this strategy is that, by focusing on certain limited categories of molecules, the limits of detection (LODs) are greatly enhanced, so that minor but important molecules can be detected. The sensitivity of the methods is greater due to the reduction of baseline noise. In addition, the combination of LC–MS-MS and focused scanning for the major group can improve identification of very minor molecular species in the particular class being analyzed (32). Taken together, a combination of multiple precursor-ion scanning (MPIS) allows quantitative monitoring of major perturbations in phospholipid composition and elucidation of the molecular heterogeneity of individual molecular species (33,34). This approach selects sequential masses in the quadrupole, fragments them in the collision cell, and then uses the rapid response of the analyzer (TOF or IT) to monitor the fatty-acyl-fragment region for each precursor mass in the sample. In untargeted experiments, if the precursors are not identified by accurately determining their masses, they can be subjected to further focused analysis in order to complete identification of the individual molecular species (28).
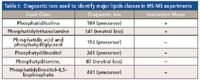
Table I: Diagnostic ions used to identify major lipids classes in MS-MS experiments
Targeted lipidomics is applied to targeted molecules by using information for both parent ions and their specific fragment ions, and an MS-MS spectrometer is also required (Figure 2). The mass spectrometer therefore runs in popular modes (selected reaction monitoring [SRM] or multiple reaction monitoring [MRM]). Logically, targeted lipidomics is often used for the analysis of lipids with well-known fragmentation patterns; however, it can also be used to scan for pairs of many theoretically expected m/z values in a survey. Ultrahigh-pressure liquid chromatography (UHPLC) TOF-MS provides an attractive alternative to current LC methods (35). Targeted lipidomics analysis is the best strategy for signalling lipids and for drugs of abuse (36). In addition, this MS strategy is highly sensitive in the analysis of a small number of individual molecular species with low LODs. MRM assays are also relatively cheap and fast to develop, and the quantitative assays are quite reproducible and accurate, allowing high-throughput analysis; thus, many lipids can be assayed in the same experiment. Recently, more than 50 lipid compounds were measured in a targeted ecosanoids lipidomics study using LC–ESI-MS-MS (with a Qtrap instrument). The analytes were measured using MRM (37). Targeted lipidomics using a nano LC–MS-MS platform and high-throughput screening of the data sets with a potent search algorithm based on fragment ion analysis has been proposed to characterize signaling lipids in the central nervous system. In this way, high-quality MS-MS spectra could be obtained even for 150 fmol of targeted lipids (38).
Shotgun Lipidomics
These three lipidomics MS strategies can be performed with or without coupling chromatographic instruments to the mass spectrometer. In the case of using direct injections of organic extracts, the analysis is referred to as shotgun lipidomics and is closely related to global and fingerprint lipidomics, two complementary lipidomics approaches. Lipid fingerprints are used to identify patterns of lipid metabolites that change in response to various stimuli. This approach, which is not intended to quantify compounds, casts a wide net and generates and tests hypotheses.
Shotgun lipidomics was originally executed on QqQ mass spectrometers, which provide sensitive and reproducible analysis with a wide dynamic quantification range (5,39). The instrumental principles and details on quantification of lipid species using QqQ instruments have been discussed elsewhere (40,41). QqQ instruments allow the acquisition of only one precursor-ion scan or one neutral-loss scan at a time, thus the analysis must be repeated to profile multiple lipid classes. When using a hybrid quadrupole time-of-flight (QqTOF) mass spectrometer, the lipidomic analysis can be performed in MPIS. In this way, MPIS enables the simultaneous acquisition of the 40–50 precursor-ion scans required in analyses such as the comprehensive characterization of eukaryotic lipidomes in a single analysis (34,42). It also permits the absolute quantification of hundreds of molecular glycerophospholipid species, glycerolipid species, sphingolipid species, and sterol lipids. Recent research discussed a novel high-throughput shotgun lipidomic platform based on 96-well, robot-assisted lipid extraction, automated sample infusion by nanoelectrospray ionization, and quantitative MPIS analysis on a QqTOF (triple-quadrupole mass spectrometer with a time-of-flight mass spectrometer replacing the third quadrupole) mass spectrometer (43).
In shotgun lipidomics, one practical means of avoiding the need for prior chromatographic systems to separate lipids into different classes is based on the physical processes present in the electrospray ion source. Given the physical factors affecting the process of selective ionization of analytes in the electrospray ion source and the different electrical properties of each of the lipid classes, the source can be used to resolve lipid-classes in a crude lipid extract into different categories based on the intrinsic electrical properties of each lipid class. This methodology for lipid-class separation is referred to as intrasource separation of lipids (44–46).
A new approach, MDMS-SL or multidimensional MS (2D MS) has recently been developed, based on the above separation process. It includes experimental conditions including ionization conditions (source temperature and spray voltage), fragmentation conditions (collision gas pressure, collision energy, or collision gas), or other modifications as additional dimensions for constructing a matrix block (25). MDMS-SL is a well-developed technology for global lipid analysis, which identifies and quantifies individual lipid molecular species directly from lipid extracts of biological samples. Using this two-dimensional ESI-MS approach, it is possible to identify and quantify the individual molecules and as a result to fingerprint most of the major and many of the minor lipid classes in cellular lipidomes directly from their chloroform extract (46). Successful studies into Alzheimer's disease also have been carried out recently by means of MDMS-SL (19).
LC–MS Analytical Lipidomics
Although many researchers claim that chromatography is not necessary and can be replaced by exploiting the unique chemistries of different lipid classes including even extremely minor lipid classes, the use of a chromatographic system is absolutely necessary to resolve certain lipidomics analysis issues. In addition, the use of LC systems before MS analysis (MS or MS-MS) is a powerful tool in the comprehensive analysis of complex lipid mixtures and in the analysis of specific categories of lipid species. The competition for ionization between molecules introduced simultaneously in the ESI source — the ion-suppression effect — also can be resolved by prior LC separation (47). Chromatography also guarantees the consistency of quantitative and qualitative results because each chromatographic peak is located in a 2-D space with a characteristic retention time versus a specific m/z. This is why LC–ESI-MS and LC–ESI-MS-MS are used in lipidomics analyses that focus on profiling prostanoids in humans (48), and the use of LC-QqTOF-MS also has been described in a recent comprehensive blood plasma lipidomics study (49).
In addition, prior chromatographic separation is absolutely necessary for the analysis of enantiomers and diastereomers of hydroxyl fatty acids and oxidized glycerolipids (50). High performance liquid chromatography (HPLC) using normal-phase or reversed-phase modes is necessary for resolution of high-molecular-weight glycerolipids before MS-MS analysis (51). The use of a chiral phase for HPLC analysis of enantiomeric lipids was reviewed recently from the perspective of a lipidomics approach (52).
Multidimensional chromatography has become more popular in recent years. This approach is especially applicable to the study of lipidomics (53), because knowledge of all molecular species of all lipid classes is sought. Recently, the two-dimensional liquid chromatography quadrupole time-of-flight mass spectrometry (2D-LC–QTOF-MS) method was developed for lipid profiling (54). One of the merits of this study was to profile different lipid classes and species that were simultaneously separated in one injection with a significantly increased sensitivity. The different lipid classes were separated on a normal-phase column in the first dimension and lipid molecular species were separated on a reversed-phase column in the second dimension, which reduced the ion suppression effects and improved the detection sensitivity. Identification of 721 endogenous lipid species from 12 lipid classes was achieved, and 415 structures were confirmed using tandem mass spectra. The other 306 lipid molecular species were identified by accurate masses.
The common strategies currently used in analytical lipidomics include direct-infusion ESI–MS and ESI–MS-MS (shotgun lipidomics), LC coupled with ESI–MS or MS-MS, and MALDI combined with FT-ICR MS (MALDI–FT-ICR MS) or TOF-MS (MALDI–TOF-MS). On the other hand, MS approaches such as SRM or SIM (applied in targeted lipidomics) usually are associated with the LC–MS platforms, and neutral-loss and precursor-ion scans are tools applied for shotgun lipidomics.
Even though shotgun experiments in lipidomics studies are well accepted, LC–MS currently is the most widely used technique in lipidomics. As a result of the predominant and powerful resolution and large peak capacity of 2D LC, 2D LC–MS will be a popular and effective technique in lipidomics analysis, especially comprehensive lipid profiling in complex biological samples (55).
MS Data Analysis in Analytical Lipidomics
The MS methods for lipid analysis provide a vast amount of MS data to identify and measure an enormous number of distinct lipid molecular species. These MS experimental data are generated within a brief interval. Processing such a quantity of MS data to extract comprehensible and meaningful information requires the use of the powerful bioinformatics tools that are used in the fields of other "omics" sciences (genomics, proteomics, and metabolomics). In addition, the structural diversity and complexity of lipids requires the development and application of new algorithms and software tools that are specifically aimed at processing data from lipid MS analyses. A variety of such software tools to process MS lipidomics data were reviewed recently (56). One such approach is the systematic study of the ion chemistry of the fragmentation of complex lipid molecular species upon collisionally activated dissociation (57,58) to identify lipid structures coupled with high-throughput database searching (58). The current state of bioinformatics and computational methods in the field of lipidomics has also recently been reviewed (59).
In the first stage of MS data lipidomics analysis, special attention must be paid to the processing of MS data. The quality of this process is essential to enable researchers to analyze and interpret lipidomics data properly. The data processing has a direct impact in terms of the quantity and quality at which lipid identification and quantification can be made. The processing of MS data can include many forms of data processing, including spectral smoothing, feature detection and peak extraction, deisotoping and deconvolution, alignment, peak identification, and statistical control analysis (60,61). Figure 3 shows a summary of a possible MS data processing workflow.
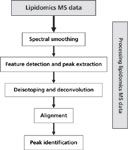
Figure 3: A general lipidomics data process workflow.
After the lipid molecular species in a sample have been identified, it is often desirable to determine their quantities. Approaches to obtaining quantitative MS data include metabolic stable isotope labeling (62) and the addition of stable-isotope-labeled internal standards (63). Sufficient resolution is required to discriminate between potentially overlapping peaks representing isobaric but distinct substances in complex biological mixtures. Despite the fact that a variety of lipid standards are available commercially for lipidomics experiments, more standards are needed for authentication and quantification purposes. Internal standards also should be used to normalize the different response factors of the particular lipid class. Nevertheless, the internal standard issue remains unresolved, because the vast number of individual molecular lipid species that can appear in the mass spectrum of a lipidomics experiment means that there is practically no free space at any m/z to situate an internal standard. A practical solution to establish the response factor of a particular class of lipids is to use external calibration (64).
The validation of both the identification and the quantification of lipids in a lipidomics experiment should represent an important stage in the quest to achieve consistent results in the way that classical analytical methods do. A probability-based scoring method was proposed recently (58) that aimed at the statistical validation of lipid molecular species identification. With respect to quantification, a commercial software program (65) has introduced the concept of a significance score, which is the number of experiments that are statistically distinguishable from the basal or control condition at a given point in time. Nevertheless, efforts have to be made in the field of analytical chemistry to put forward rigorous procedures to validate the identification and quantification of lipids in lipidomics experiments.
The final analysis of a lipididomics study involves converting the MS data obtained after processing into the biological information required to answer the question or issue that originated the study. Usually, the results of MS data processing from a control condition are compared with the results obtained from samples under a different condition. To compare the results, chemometrics approaches such as principal component analysis (PCA) and partial least squares (PLS) are applied. Databases and search algorithms are being developed to integrate chromatographic and MS data of cellular lipidomics (66–69).
Main Online Resources for the Study of Lipidomics
A range of well-established resources on lipids and lipidomics are available on the Internet. Several of these platforms normally require MS data (70).
The European Lipidomic Initiative from Europe (71), The Lipid Mass Consortium from La Jolla, California (72), and The Lipid Bank from Japan (73) are promising platforms that should help to standardize lipidome related nomenclatures and establish and harmonize lipid metabolic pathways and tools at a worldwide level.
The European Initiative has launched the European Lipidomics Expertise Platform (70). This platform aims to promote research activities in the field of lipidomics, so as to understand the outstanding functional importance of structural lipids and their interaction with proteins, the function of biologically active lipids in life sciences and human health and disease, and the genome of the lipidome.
The Lipid Metabolites and Pathways Strategy (Lipid MAPS) is a consortium that focuses on the lipid section of the metabolome by developing an integrated metabolomic system capable of characterizing the global changes in lipid metabolites. The Lipid MAPS initiative also proposes the adoption of MS as the instrumental platform to investigate lipidomics because it has already demonstrated its capacity to identify and quantify an important number of known individual lipid molecular species and search for new lipids affecting biological systems.
The Lipid Bank is, primarily, a database that contains factual data, such as lipid names, chemical and physical properties, biological activities, metabolism, and genetic information. It also contains MS spectral data of lipids.
Fields of Application of Analytical Lipidomics
As stated throughout this article, MS is now the preferred instrumental analytical platform for determining lipids in a comprehensive analysis of the lipidome in all the areas in which lipidomics is studied. Research areas that benefit from MS analytical lipidomics include medicine, pharmaceuticals, nutrition, and food–agriculture.
Within the context of functional lipidomics in plants, ESI-MS-MS-based lipid plant profiling has been used to identify lipid metabolic pathways that play a part in plant development and stress responses, to specify the roles of particular genes and enzymes in plant responses to environmental cues, to determine the lipid species that serve as the substrates and products of specific enzymes, and to identify lipid-metabolizing enzymes that are involved in a variety of plant processes (74).
There are many functional lipidomics studies of mammals. One of the most recent used LC–ESI-QTOF-MS in lipidomics metabolism analysis of the endogenous cannabinoid anandamide (N-arachidonylethanolamide) (75). In this work, a major technical issue in LC–MS analysis of lipids (poor ionization efficiency of endocannabinoid molecules) was overcome by adding silver to the mobile phase.
MS lipidomics appears to have great potential applications in medicine, including diagnosis, therapy, and analysis of the mechanisms underlying various diseases or other pathophysiological conditions (76). Overviews of this subject have been published elsewhere (77,78). One of the latest studies presented in this field deals with the study of intracellular Singapore grouper iridovirus (79). The analysis was performed using a MALDI-TOF–TOF MS-MS platform, and the authors claim that this work was the first research detailing the lipidome and lipid–protein interactions of an unenveloped virus.
The pharmaceutical industry can benefit from biomarker and drug lipidomics approaches (80). These can be used to discover new drugs through the discovery of new metabolites and mechanisms of action, or to identify possible toxic effects of the drug. In addition, as biomarkers, the lipidomic approach can promote early detection of diseases and facilitate the development of diagnostic kits. A recent review emphasized its applications in disease biomarker discovery (81). It shows that the MS instruments, coupled or not to a chromatography system (including UHPLC–MS instruments), are the most commonly used techniques for disease biomarker discovery. It has also been shown that ESI is the most popular ionization interface for these purposes (82).
Lipidomics approaches are being applied to investigate bioactive compounds like eicosanoids (83) with key roles in innate immunity and inflammation. MS offers selectivity and sensitivity to study fatty acid remodeling within individual glycerophospholipid species and the role of phospholopase A2 in this process. The analytical method for using ESI-MS-MS or HPLC–ESI-MS-MS depends on the information that the researcher wishes to obtain.
Nutrition and food lipidomics are related to changes in the lipidome as the result of a specific diet. The first study to investigate the effects of probiotics intervention on global lipidomics profiles in humans was performed using UHPLC–MS, and a total of 407 lipids were identified, corresponding to 13 different lipid classes (84). A recent lipidomics study demonstrated that dietary carbohydrate modification alters serum metabolic profiles in individuals with metabolic syndrome (85). In this case, chromatographic separation was also performed using UHPLC coupled to QTOF-MS.
As a result of the highly valuable information that can be obtained by lipidomics approaches in a wide range of areas (some cited above), the number of publications on MS lipidomics is growing fast. Table II shows recent literature with specific examples of lipidomics approaches in different fields; the MS platform used in each case is highlighted. This table is an update of another table taken from a previous, more general review of lipidomics (86).

Table II: Fields of application of analytical lipidomics
Conclusion
The present review has aimed to provide a view of the current application of MS in the analysis of the lipidome. During the last few years, the number of publications concerning lipidomics has grown significantly, showing an important interest of the scientific community in this newly emerged "omic" field. Currently, novel MS lipidomics technology enables an unprecedented power in lipid research in areas such as medicine, pharmaceutical development, nutrition, and agriculture. Thus, the collaboration of researchers from all these areas will benefit the future progress of lipidomics, including efforts from analytical chemistry to resolve problems related with the development and validation of the MS lipidomic analytical methods.
Acknowledgments
The authors would like to thank to the Andalusia Regional Government (Consejería de Innovación, Ciencia y Empresa, projects P07-FQM-02667 and P09-FQM-5469) for financial assistance. This work has also been partially supported for the European Regional Development Funds (ERDF).
Luis Cuadros-Rodríguez, Alegría Carrasco-Pancorbo, and Natalia Navas Iglesias are professors in the Department of Analytical Chemistry at the University of Granada, Granada, Spain.
References
(1) G.R. Fenwick, J. Eagles, and R. Self, Biomed. Mass Spectrom. 10, 382–386 (1983).
(2) F.F Hsu and J. Turk in Modern Methods for Lipid Analysis by Liquid Chromatography/Mass Spectrometry and Related Techniques, W.C. Byrdwell, Ed. (AOCS Press, Urbana, Illinois, 2005), pp. 61–178.
(3) X. Han and R.W. Gross, J. Lipid Res. 44, 1071–1079 (2003).
(4) M. Pulfer and R.C. Murphy, Mass Spectrom. Rev. 22, 332–364 (2003).
(5) X. Han and R.W. Gross, Mass Spectrom. Rev. 24, 367–412 (2003).
(6) J. Schiller, R. Suss, B. Fuchs, M. Muller, O. Zschornig, and K. Arnold, Front. Biosci. 12, 2568–2579 (2007).
(7) F. Spencer, M. Lagarde, A. Géloën, and M. Record, Eur. J. Lipid Sci. Technol. 105, 481–482 (2003).
(8) G. Meer, B.R. Leeflang, G. Liebisch, G. Schimitz, and F.M. Goñi, Methods Enzymol. 432, 213–232 (2007).
(9) A. Carrasco-Pancorbo, N. Navas-Iglesias, and L. Cuadros-Rodriguez, Trac. Trend Anal. Chem. 28, 263–278 (2009).
(10) L.D. Roberts, G. McCombie, C.M. Titman, and J.L. Griffin, J. Chromatogr. B 871, 74–181 (2008).
(11) W. Hou, H. Zhou, F. Elisma, S.A. Bennett, and D. Figeys, Brief Funct. Genomics Proteomics 7, 395–409 (2008).
(12) C. Hu, R. Heijden, M. Van der Wang, J. Van der Greef, T. Hankemeier, and G. Xu, J. Chromatogr. B 877, 2836–2846 (2009).
(13) T. Seppanen-Laakso and M. Oresic, J. Mol. Endocrinol. 42, 185–190 (2009).
(14) B Fuchs, .R. Sü, and J. Schiller, Lipid Res. 49, 450–475 (2010).
(15) P.D. Rainville, C.L. Stumpf, J.P. Shockor, R.S. Plumb, and J.K. Nicholson, J. Proteome Res. 6, 552–559 (2007).
(16) J. Ding, C.M. Sorensen, N. Jaitly, H. Jiang, D.J. Orton, M.E. Monroe, R.J. Moore, R.D. Smith, and T.O Metz, J. Chromatogr. B 871, 243–252 (2008).
(17) C. Hu, J. van Dommelen, R. van der Heijden, G. Spijksma, T.H. Reijmers, M. Wang, E. Slee, X. Lu, G. Xu, J. van der Greef, and T. Hankemeier, J. Proteome Res. 7, 4982 (2008).
(18) Z. Cui and M.J. Thomas, J. Chromatogr. B 877, 2709–2715 (2009).
(19) X. Han, Biochim Biophys. Acta 1801, 774–783 (2010).
(20) J.B. Fenn, M. Mann, J.B. Meng, S.F. Wong, and C.M. Whitehouse, Science 246, 64–71 (1989).
(21) K. Tanaka, H. Waki, Y. Ido, S. Akita, Y. Yoshida, and T. Yoshida, Rapid Commun. Mass Spectrom. 2, 151–153 (1988).
(22) A. Cho and D. Normile, Science 298, 527–528 (2002).
(23) S. Milne, P. Ivanova, J. Forrester, and H.A. Brown, Methods 39, 92–103 (2006).
(24) J Schiller, .R. Su, J. Arnhold, B. Fuchs, J. Le ig, M. Muller, M. Petkovic, H. Spalteholz, O. Zschornig, and K. Arnold, Prog. Lipid Res. 43, 449–488 (2004).
(25) X. Han and R.W. Gross in Lipid Analysis and Lipidomics: New Techniques and Applications, R.E. McDonald, Ed. (AOCS Press, Urbana, Illinois, 2006), pp. 51–71.
(26) J.J. Jones, S. Borgmann, R.M.O. Brien, and C.L. Wilkins, Anal. Chem. 78, 3062–3071 (2006).
(27) C. Wang, H. Kong, Y. Guan, J. Yang, J. Gu, S. Yang, and G. Xu, Anal. Chem. 77, 4108–4116 (2005).
(28) D. Schwudke, J.T. Hannich, V. Surendranath, V. Grimard, T. Moehring, L. Burton, T. Kurzchalia, and A. Shevchenko, Anal. Chem. 79, 4083–4093 (2007).
(29) M. Ishida, T. Yamazaki, T. Houjou, M. Imagawa, A. Harada, K. Inoue, and R. Taguchi, Rapid Commun. Mass Spectrom. 18, 2486–2494 (2004).
(30) T. Houjou, K. Yamatani, M. Imagawa, T. Shimizu, and R. Taguchi, Rapid Commun. Mass Spectrom. 19, 654–666 (2005).
(31) G. Stubiger and O. Belgacem, Anal. Chem. 79, 3206–3213 (2007).
(32) R. Taguchi, T. Houjou, H. Nakanishi, T. Yamazaki, M. Ishida, M. Imagawa, and T. Shimizu, J. Chromatogr. B 823, 26–36 (2005).
(33) K. Ekroos, I.V. Chermushevich, K. Simons, and A. Shevchenko, Anal. Chem. 74, 941 (2002).
(34) K. Ekroos, C.S. Ejsing, U. Bahru, K. Simons, and A. Shevchenko, J. Lipid Res. 44, 2181–2192 (2003).
(35) P.D. Rainville, C.L. Stumpf, J.P. Shockcor, R.S. Plumb, and J.K. Nicholson, J. Proteome Res. 6, 552–558, (2007).
(36) R.S. Rapaka, D. Piomelli, S. Spiegel, N. Bazan, and E.A. Dennis, Prostag. Oth. Lipid M.77, 223–234 (2005).
(37) M. Sanak, A. Gielicz, K. Nagraba, M. Kaszuba, J. Kumik, and A. Szczeklik, J. Chromatogr. B 878, 1796–1800 (2010).
(38) B. Tan, Y.W. Yu, M.F. Monn, H.V. Hughes, D.K. O'Dell, and J.M. Walker, J. Chromatogr. B 877, 2890–2894 (2009).
(39) G. Liebisch, B. Lieser, J. Rathenberg, W. Drobnik, and G. Schmitz, Biochim. Biophys. Acta. 1686, 121–128 (2004).
(40) B. Brugger, G. Erben, R. Sandhoff, F.T. Wieland, and W.D. Lehmann, Proc. Natl. Acad. Sci. U.S.A. 94, 2339–2344 (1997).
(41) M. Koivusalo, P. Haimi, L. Heikinheimo, R. Kostiainen, and P. Somerharju, J. Lipid Res. 42, 663–672 (2001).
(42) E. Ejsing, E. Duchoslav, J. Sampaio, K. Simons, R. Bonner, C. Thiele, K. Ekroos, and A. Shevchenko, Anal. Chem. 78, 6202–6214 (2006).
(43) M. Stahlmana, C.S. Ejsingb, K. Tarasovc, J. Permana, J. Boréna, and K. Ekroosc, J. Chromatogr. B 877, 2664–2672 (2009).
(44) M. Pulfer and R.C. Murphy, Mass Spectrom. Rev. 22, 81–152 (2003).
(45) D.J. Schiller, R. Suss, B. Fuchs, M. Muller, O. Zschornig, and K. Arnold, Front. Biosci. 12, 2568–2579 (2007).
(46) X. Han, J. Yang, H. Cheng, H. Ye, and R.W. Ross, Anal. Biochem. 330, 317–331 (2004).
(47) J.X. Shen, R.J. Motyka, J.P. Roach, and R.H. Hayes, J. Pharm. Biomed. Anal. 37, 359–367 (2005).
(48) J.H. Durn, K.M. Marshall, D. Farrar, P. O'Donovan, A.J. Scally, D.F. Woodward, and A. Nicolaou, Prostag. Leukotr. Ess. 82, 21–26 (2005).
(49) K. Sandra, A.S. Pereira, G. Van Hoenacker, F. David, and P. Sandra, J. Chromatogr. A 1217, 4087–4099 (2010).
(50) A. Kuksis and O. Sjovall in Lipid Analysis and Lipidomics: New Techniques and Applications, R.E. McDonald, Ed. (AOCS Press, Urbana, Illinois, 2006), pp 109–156.
(51) W.C. Birdwell and W.E. Neff, J. Chromatogr. A 905, 85–102 (2001).
(52) A. Kuksis and Y. Itabashi in Lipid Analysis and Lipidomics: New Techniques and Applications, R.E. McDonald, Ed. (AOCS Press, Urbana, Illinois, 2006), pp. 73–108.
(53) N. Zehethofer and D.M. Pinto, Anal. Chim. Acta. 627, 62–70 (2008).
(54) H. Nie, R. Liu, Y. Yang, Y. Bai, Y. Guan, D. Qian, T. Wang, and H. Liu, J. Lipid Res. 51, 2833–2844 (2010).
(55) M. Li, Z. Zhou, H. Nie, Y. Bai, and H. Liu, Anal. Bioanal. Chem. 399, 243–249 (2011).
(56) H. Songa, J. Ladensonb, and J. Turka, J. Chromatogr. B 877, 2847–2854 (2009).
(57) F.F. Hsu and J. Turk, in Modern Methods for Lipid Analysis by Liquid Chromatography Mass Spectrometry and Related Techniques, William Craig Byrdwell, Ed. (AOCS Publication, Urbana, Illinois, 2005).
(58) H. Song, F.F. Hsu, J. Ladenson, and J. Turk, J. Am. Soc. Mass Spectrom. 18, 1848–1858 (2007).
(59) P.S. Niemela, S. Castillo, M. Sysi-Aho, and M. Oresic, J. Chromatogr. B 877, 2855–2862 (2009).
(60) M. Katajamaa and M. Oresic, J. Chromatogr. A 1158, 318–328 (2007).
(61) V. Pravdova, B. Walczak, and D.L. Massart, Anal. Chim. Acta. 456, 77–92 (2002).
(62) M.D. Leavell and J.A. Leary, Anal. Chem. 78, 5497–5503 (2006).
(63) R.C. Murphy, P.F. James, A.M. McAnoy, J. Krank, E. Duchoslav, and R.M. Barkley, Anal. Biochem. 366, 59–70 (2007).
(64) C. Wolf and P.J. Quinn, Prog. Lipid Res. 47, 15–36 (2008).
(65) J.S. Forrester, S.B. Milne, P.T. Ivanova, and H.A. Brown, Mol. Pharmacol. 65, 813–821 (2004).
(66) Y. Lu, S. Hong, E. Tjonahen, and C.N. Sherhan, J. Lipid Res. 34, 46790 (2005).
(67) P. Haimi, A. Uphoff, M. Hermansson, and P. Somerharju, Anal. Chem. 78, 8324–8331 (2006).
(68) M. Sysi-Aho, A. Vehtari, V. Velagapudi, J. Westerbacka, L. Yetukuri, R. Bergholm, M.R. Taskinen, H. Yki-Järvinen, and M. Oresic, Bioinformatics 23, i519–i528 (2007).
(69) L. Yetukuri, M. Katajamaa, G. Medina-Gomez, T. Seppänen-Laakso, A. Vidal-Puig, and B.M.C. Oresic, Syst. Biol. 23, 1:12 (2007).
(70) http://www.lipidomics.net/deliverables/expertise
(71) http://www.lipidomics.net/
(72) http://www.lipidmaps.org/
(73) http://lipidbank.jp/
(74) R. Welti and X. Wang, Curr. Opin. Plant Biol.7, 1–8 (2004).
(75) E.A. Placzeka, B.R. Cooperb, A.T. Placzeka, J.A. Chesterc, J.V. Davissona, and E.L. Barkera, J. Pharmaceut. Biomed. 53, 567–575 (2010).
(76) M. Oresic, S. Simell, M. Sysi-Aho, M. Sysi-Aho, K. Näntö-Salonen, T. Seppänen-Laakso, and V. Parikka, J. Exp. Med. 205, 2975–84 (2008).
(77) A. Uphoff, M. Hermansson, P. Haimi and P. Somerharju, in Analysis of Complex Lipidomes, Medical Applications of Mass Spectrometry, K. Vékey, A. Telekes, and A. Vertes, Eds. (Elsevier, Amsterdam, 2008), pp. 223–249.
(78) M.B. Khalil, W. Hou, H. Zhou, F. Elisma, L.A. Swayne, A.P. Blanchard, Z. Yao, S.A.L. Bennett, and D. Figeys, Mass Spectrom. Rev . 29, 877–929 (2010).
(79) J. Wu, R. Chan, M.R. Wenk, and C. Hew, Virology 399, 248–256 (2010).
(80) X. Han, Curr. Opin. Mol. Ther. 9, 586–591 (2007).
(81) C. Hua, R. Heijden, M. Greef, T. Hankemeier, and G. Xua, J. Chromatogr. B 877, 2836–2846 (2009).
(82) A.N. Hunt, M. Macken, G. Koster, J.A. Kohler, and A.D. Postle, Advan. Enzyme Regul. 48, 74–87 (2008).
(83) D. Balgoma, O. Montero, M.A. Balboa, and J. Balsinde, Biochimie 92, 645–50 (2010).
(84) R.A. Kekkonen, M. Sysi-Aho, M. Seppänen-Laakso, I. Julkunen, H. Vapaatalo, M. Orešic, and M, Korpela, World J. Gastroenterol 4, 3188–3194 (2008).
(85) M. Lankinen, U. Schwab, and P.V. Gopalacharyulu, Nutr. Metab. Cardiovasc. Dis. 20, 249–257 (2010).
(86) N. Navas-Iglesias, A. Carrasco-Pancorbo, and L. Cuadros-Rodrígez, Trac. Trend Anal. Chem. 28, 393–403 (2009).
(87) H. He, C.A. Conrad, C.L. Nilsson, Y. Ji, T.M. Schaub, A.G. Marshall, and M.R. Emmett, Anal. Chem. 79, 8423–8430 (2007).
(88) H. Ogiso, T. Suzuki, and R. Taguchi, Anal. Biochem. 378, 43–52 (2008).
(89) H.A. Boumann, M.J.A. Damen, C. Versluis, A.J.R. Heck, B. Kruijff, and A. de Kroon, Biochemistry 42, 3054–3059 (2003).
(90) R. Schneiter, B. Brugger, R. Sandhoff, G. Zeilinig, A. Leber, M. Lampl, K. Athenstaedt, C. Hrastnik, S. Eder, and G. Daum, J. Cell Biol. 146, 741–754 (1999).
(91) A.D. Postle, D.C. Wilton, A.N. Hunt, and G.S. Attard, Prog. Lipid Res. 46, 200–224 (2007).
Luis Cuadros-Rodríguez, Alegría Carrasco-Pancorbo, and Natalia Navas Iglesias are professors in the Department of Analytical Chemistry at the University of Granada, Granada, Spain.

HPLC 2025 Preview: Fundamentally Speaking (Part 2)
May 14th 2025Michael Lämmerhofer from the Institute of Pharmaceutical Sciences, University of Tübingen, Germany, spoke to JFK Huber Lecture Award winner of 2024 Torgny Fornstedt, professor in analytical chemistry and leader of the Fundamental Separation Science Group, Karlstad University, Sweden, about his pioneering work in high performance liquid chromatography (HPLC) with a focus on fundamentals, ion-pair chromatography, and oligonucleotide applications.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











