Magnetic Nanoparticles for Solid-Phase Extraction
LCGC Europe
Magnetic nanoparticles (m-NPs) are becoming important in analytical chemistry as sorbents in dispersive solid-phase extraction (d-SPE) because they simplify the extraction process and save time as a result of their isolation from the sample matrix by an external magnetic field. Many synthetic processes have been developed to fabricate these nanomaterials and an additional coating step is usually included at the end of the synthesis to improve their stability and to avoid the formation of agglomerates. A wide variety of coatings have been used for this purpose to improve selectivity. This manuscript provides an overview of the different synthesis methods, coatings, and applications of m-NPs as sorbents in d-SPE.
Photo Credit: Sheri Neva/Getty Images
Javier González-Sálamo1, Antonio V. Herrera-Herrera1, Chiara Fanali2, and Javier Hernández-Borges1, 1Departamento de Química, Unidad Departamental de Química Analítica, Facultad de Ciencias, Universidad de La Laguna (ULL), San Cristóbal de La Laguna, Tenerife, Islas Canarias, España, 2Integrated Center of Research, Campus Bio-Medico University, Rome, Italy.
Magnetic nanoparticles (m-NPs) are becoming important in analytical chemistry as sorbents in dispersive solid-phase extraction (d-SPE) because they simplify the extraction process and save time as a result of their isolation from the sample matrix by an external magnetic field. Many synthetic processes have been developed to fabricate these nanomaterials and an additional coating step is usually included at the end of the synthesis to improve their stability and to avoid the formation of agglomerates. A wide variety of coatings have been used for this purpose to improve selectivity. This manuscript provides an overview of the different synthesis methods, coatings, and applications of m-NPs as sorbents in d-SPE.
Nanomaterials are important in many branches of chemistry, including analytical chemistry. Nanoparticles (NPs) consisting of different shapes and materials with different physical and chemical properties are currently being used as stationary phases and sorbents in separation science.
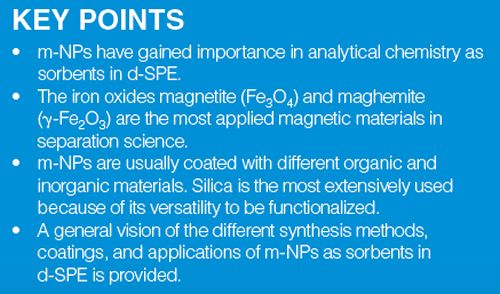
An important group of NPs in analytical chemistry have a permanent magnetism (ferromagnetism) or can be attracted by a magnetic field without retaining residual magnetism after the magnetic field is eliminated (superparamagnetism) (Figure 1). Both types of magneticâNPs (m-NPs) have successfully been used as solid-phase extraction (SPE) sorbents, because they aid the extraction procedure and save time in sample preparation. In conventional SPE the sorbents are packed into cartridges, disks, or pipette tips, whereas in magnetic-SPE (m-SPE), which can also be classified as dispersive-SPE (d-SPE), the sorbent is added directly to the sample. After analyte sorption, the sorbent is retained on one side of the extraction recipient with an appropriate magnet. For elution, a suitable solvent is added and the sorbent is dispersed and retained with the magnet. In general, m-SPE is simple, rapid, and efficient (d-SPE without m-NPs require the use of high-speed centrifugation or filtration).
The development of novel sorbents with high sorption capacity, high surface area, and stability is an active research area in analytical chemistry. The fabrication of m-NPs frequently requires three different steps: the synthesis of the m-NP itself, the coating of this magnetic core, and, very frequently, the further modification of the surface to obtain the desirable interaction. This article aims to provide an overview of m-NPs synthetic procedures, including their combination or coating with different organic or inorganic materials and their most relevant current applications.
m-NPs Synthesis
m-NPs are commonly composed of metals like iron, nickel, and cobalt, their oxides, spinel-type ferromagnets, including MgFe2O4, MnFe2O4, and CoFe2O4, as well as alloys such as CoPt3 and FePt. They have ferro- or superpara-magnetic properties in a wide range of sizes.
The iron oxides magnetite (Fe3O4) and maghemite (γ-Fe2O3) are by far the most applied magnetic materials in separation science because of the existence of multiple and simple procedures of fabrication (2); their superparamagnetic character which makes then easy to manipulate as a result of their magnetic behaviour at room temperature; their small size and high surface area (providing great extraction capacity and relatively low extraction times); and their low toxicity (3).
Different synthetic procedures have been developed to obtain iron oxide m-NPs, although most of them can also be used to fabricate different m-NPs by simply changing the precursor salt. The m-NPs obtained by these procedures are available in a wide range of sizes, from nanometer-size (1–100 nm) to microparticles behaviour because such characteristic allows an easier manipulation. Such synthetic procedures include: chemical co-precipitation; solvothermal/hydrothermal reactions; thermal decomposition; microemulsion/nanoemulsion based synthesis; metal reduction;
flow injection methods and aerosol/vapour phase procedures (2,4).
Maghemite can also be easily obtained by further oxidation of magnetite by, for example, dissolving it in an acidic medium (with the eventual addition of iron [II] nitrate) and heating it to 90–100 °C (5), or by exposing it to air for a long period of time (via cation diffusion) (6). In fact, the oxidation of magnetite to maghemite is a potential advantage because this last material is ferrimagnetic (the macroscopic characteristics are similar to ferromagnetic materials, the only difference is the origin of magnetic moments) and the particles obtained are chemically stable in both alkaline and acidic medium (4).
In co-precipitation procedures (based on both nucleation and growth of an iron hydroxide nuclei [2]) Fe2+ and Fe3+ salts (mainly FeCl3·6H2O and FeCl2·4H2O although sulphates and nitrates can also be used) are mixed, heated at about 75–80 °C (this step could be avoided), and precipitated by adding aqueous ammonia or another alkaline solution (3), because pH should be in the range of 8 to 14 to ensure complete precipitation. By varying the experimental conditions (ionic strength, pH, molar ratio of reactants, and temperature), the diameter, magnetic responsiveness, and surface properties of NPs can be controlled. In fact, different experimental conditions leading to m-NPs of different size, shape, and composition can be frequently found in the literature, but once the experimental conditions are established the procedure is highly reproducible. However, this method tends to produce a wide particle size distribution, which results in non-ideal magnetic behaviour for many applications. The magnetic saturation (Ms) of magnetite NPs obtained in this way is experimentally determined to be in the range 30–50 emu/g. Such a procedure has also been applied to magnetic materials such as those made from cobalt (7–9), nickel-zinc ferrite (10), or manganese (11).
The solvothermal approach (which allows ultrafine m-NPs to be obtained) frequently involves mixing FeCl3·6H2O, ethylene glycol, and sodium acetate (12,13), and then heating for a specific period of time (temperatures higher than 200 °C could be necessary [12]) after which it is necessary to cool at room temperature. Ethylene glycol acts as solvent and reductant while sodium acetate acts as an assistant reductant and electrostatic stabilizer. In some cases, to improve the synthesis and to speed up the procedure, the solvothermal approach has been assisted by microwaves (14,15). Microwave irradiation provides a burst of hotâspots for uniform seeding, which accelerates the formation of magnetic nanocristals. Fe3O4 NPs fabricated by this method showed higher saturation magnetization as a result of the smaller particle size (14).
In thermal decomposition, which is also applicable to Co and Ni m-NPs, a metal carbonyl, a low-valent metallic alkene, polyene metal complexes, and metal oleates precursors are treated with high-boiling point organic solvents containing stabilizing surfactants at high temperatures and pressures in reactors or autoclaves (2,4). This method was inspired by the synthesis of highâquality semiconductor nanocrystals and oxides in non-aqueous media. The ratios of starting materials (including organometallic compounds, surfactants, and solvents) as well as the reaction temperature, time, and ageing period are crucial for the control of morphology and size (4). Despite the fact that these m-NPs have a narrow size distribution (with good size control) and a good crystallinity, their high cost and the use of toxic organic reagents discourage the use of the procedure.
When metal reduction is used, metallic salts are treated with reducing agents in the presence of a surfactant or by electrochemical reduction (2,4). Precursors used in this approach are stable salts such as oxides, nitrates, chlorides, and acetates, in contradistinction to thermal reduction, which uses organometallic precursors.
These methods can also be performed in microemulsions to better control the particle size (2,4). However, a wide variety of sizes and shapes and a low yield are frequently obtained, in addition to the high volumes of solvents required.
Flow injection methods, which are highly reproducible, imply the continuous (or segmented) mixing of reagents under a laminar flow (2). In the aerosol/vapour phase techniques, the precursors are continuously reduced
in an aerosol or gas state in a suitable reactor (2). Because of the rapid expansion of the gas phase, the growth and agglomeration of particles is partially diminished.
It should be noted that the synthesis of m-NPs has two principal problems: m-NPs tend to form agglomerates (to reduce the energy associated with the high surface-area to-volume ratio of the nanosized particles) and are also chemically active (easily oxidizable in air), which results in a loss of magnetism and dispensability. Moreover, the lack of selectivity of metallic m-NPs, derived from their high extraction capacity, makes them inappropriate for complex matrices. As previously mentioned, a suitable strategy to avoid these situations consists of coating or grafting with an inorganic (silica, carbon - including graphene and carbon nanotubes [CNTs] - alumina, etc.) or organic (surfactants or polymers) layer (16). This will be described in the next section.
m-NPs Combination/Modification With Other Materials
One of the most common strategies used to avoid the formation of agglomerates and to improve the stability of m-NPs involves their protection by an impenetrable layer such as a core–shell structure (4). In analytical applications, this protective coating, which can be inorganic or organic, is not only used to stabilize and protect m-NPs but also for its further functionalization, because the modified particles are frequently customized to improve specific properties (4) (Figure 2). In this sense, there are currently a wide variety of inorganic and organic coating materials available. The most common will be described in the following sections (m-NPs characteristics - surface area (s.a), diameter(d) and Ms - are provided when available).
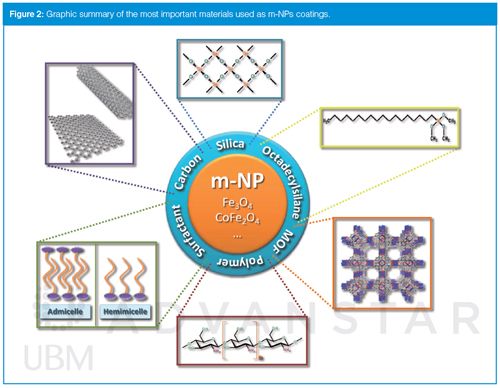
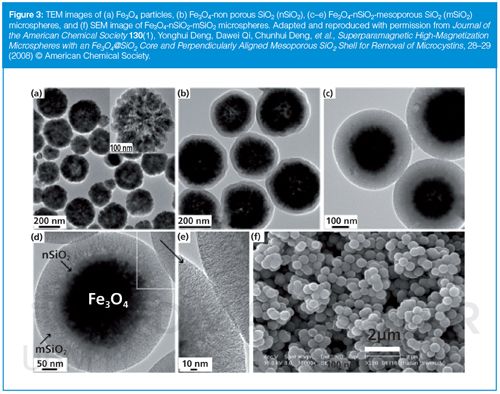
Silica-Coated m-NPs: Silanization is one of the most commonly used methods to modify m-NPs surface. This may be because of silica’s low price, chemical inertness, and mechanical, chemical, and thermal stability (3,16). In fact, the technology of coating with silica can even allow the combination of shells with different properties. This fact is verified in Figure 3 where a magnetic Fe3O4 core was surrounded by a non-porous silica layer (used to protect the magnetite) and then a mesoporous silica shell with a high surface area (365 m2/g) was deposited (17). When a m-NP is coated by silica, the presence of abundant silanol groups on its surface makes an easy surface modification by other organic or inorganic functional groups or other materials possible (3,16). In this sense, it is quite common to use silica-bonded compounds containing hydroxyl, thiol, or amino groups in their structures to bind heavy metal ions acting like a coupling agent to form a coordination compound. Some of these species may be γ-mercaptopropyltrimethoxysilane (γ-MPTMS) (18) - d: 50–70 nm; Schiff bases (19) - s.a: 106.4 m2/g, d: 38 nm; salicylic acid (20) - s.a: 41.62 m2/g, d: 58–73 nm; or iminodiacetic acid (21) - s.a: 87.7 m2/g, d: 200 nm. It is also possible to find m-NPs functionalized with diphenyl groups (22) - s.a: 150 m2/g, d: 200 nm, Ms: 16 emu/g; cyclodextrins (23) - s.a: 60.08 cm2/g, d: 200 nm, Ms: 34.3 emu/g; and even
with metalâorganic frameworks (MOFs) (24) - Ms: 21 emu/g; surfactants (25) - Ms: 69.64 emu/g; or ionic liquids (ILs) (26).
Octadecylsilane (ODS)-Modified m-NPs: ODS coatings have been widely used in sample preparation because they offer an excellent adsorption and separation capacity even within complex matrices (16). One of the most promising and advanced composite materials is SiO2âC18âcoated m-NPs, which are essentially a modification of the silica-coated m-NPs discussed previously. However, in recent years different modifications of this composite have also emerged for analytical purposes, including C18-functionalized mesoporous silica shell on the surface of a Fe3O4âSiO2 core (27) - s.a: 331 m2/g, d: 200 nm, Ms: 7.35 emu/g; silica-coated m-NPs functionalized with both C18 and -NH2 groups (28) - s.a: 69.27 m2/g, d: 25 nm, Ms: 44 emu/g; or titanate nanotube-coated m-NPs functionalized with C18 groups and then coated with an alginate-polymer cage (29) - s.a: 163–223 m2/g, d: 30–50 nm, Ms: 7.3 emu/g. These modifications have improved the extraction capacity and selectivity of the sorbent, enhancing the recoveries and reducing matrix effects.
Carbon-Coated m-NPs: Most studies on m-NP coatings have been focused on the development of silica or polymers as protective shells, as will be shown later. However, in recent years, new carbon-protected m-NPs have been synthesized because of their advantages over the previously mentioned coatings, such as higher chemical and thermal stability, biocompatibility, and possibility of surface modification or pore creation (4,16). Carbon used to modify the surface of the m-NPs can be found in various forms:
Activated Carbon, Graphitized Carbon Black, or Porous Graphitic Carbon-Coated m-NPs: These carbon forms have a good adsorption capacity, but they may cause desorption problems because of irreversible adsorption of some analytes. However, these adverse effects should be overcome by using them as coatings of m-NPs (30) - s.a: 71 m2/g, Ms: 43.75 emu/g. This may be one of the reasons why numerous works using these kind of coatings have been reported in recent years (31,32) - s.a: 315 m2/g, d: 3–6 µm, Ms: 27.99 emu/g. Moreover, some functionalized carbon-coated m-NPs have already been used (33)
- d: 200 nm.
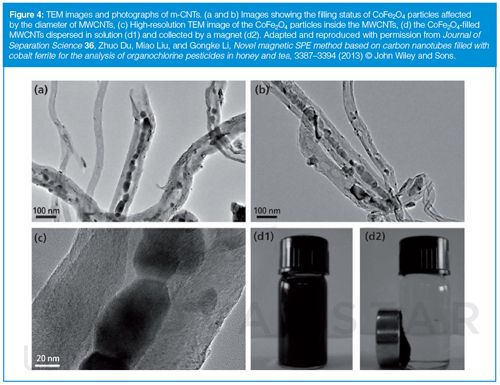
CNTs-Based m-NPs: As a result of their high surface area, their ability to establish π-π interactions, and excellent chemical, thermal, and mechanical stability, CNTs have been used as sorbents in sample treatment (34,35). However, their poor solubility in water and organic solvents and the difficulty involved in collecting them from their dispersed media sometimes complicates their practical applicability (16). This problem can be overcome by combining their attractive surface properties and characteristic structures with magnetic properties of certain NPs. Occasionally, the surface of CNTs should be funtionalized to provide certain properties (16,36). The combination of both nanomaterials can be performed by filling CNTs with m-NPs (36) (Figure 4) - Ms: 0.77 emu/g; or by deposition of m-NPs onto the surface of the CNTs (37) - Ms: 24.9 emu/g. With regards to the different types of combinations between CNTs and m-NPs (filling or deposition) most applications of CNTs-coated m-NPs in SPE have been reported using multi-walled (MW) CNTs (38,39). On occasion they have been used in combination with polymers (40) or ILs (41) - s.a: 171.5 m2/g, Ms: 52.52 emu/g, among others.
Graphene-Based m-NPs: Discovered in 2004, graphene has attracted the attention of the scientific community ever since. This carbon’s allotrope has many unique features, including a two-dimensional planar structure coupled with one-atom thickness, extraordinary electrical, thermal, and mechanical properties, and a large surface area, which is evidence of its high sorption capacity (16,42). As with CNTs, graphene’s large delocalized π-electron system favours a strong π-stacking interaction with benzene-based organic compounds (16). Therefore, the combination of the magnetic properties of the m-NPs with the good adsorption characteristics of graphene makes this composite another alternative as sorbent in SPE. In this sense, it is possible to find many works that make use of both graphene-based m-NPs (42–44) - s.a: 186 m2/g (44), d: 5–200 nm; and graphene oxide-based m-NPs (45,46) - s.a: 145.8 m2/g (45), Ms: 17.9–44.1 emu/g. Some authors have reported the use of grapheneâbased m-NPs modified with different molecules; for example, cyclodextrins (47) combined with ILs (48) - Ms: 35.93 emu/g; or graphene immobilized onto silicaâcoated m-NPs (49) - d: 400 nm, Ms: 21 emu/g.
Surfactant-Coated m-NPs: Surface-active agents or surfactants have been widely used in sample preparation for the extraction and pre-concentration of a wide number of organic and inorganic compounds (50). In recent years, the advantages demonstrated by both m-NPs in sample treatment and the adsorption capacity of some ionic surfactants (for example, sodium dodecyl sulphate [SDS], cetyltrimethylammonium bromide [CTAB], cetylpyridinium chloride, etc.) onto inorganic surfaces have led to their combination to create a new type of sorbent for SPE with a high surface area, high chemical stability, and good magnetic separability (50). Such adsorption leads to the simultaneous formation of hemimicelles and admicelles on the surface of m-NPs depending on the surfactant concentration. This allows a sorbent with different mechanisms of retention to be obtained: hydrophobic (hemimicelles) and ionic (admicelles) (16).
Currently, there is a wide variety of surfactants that can be used in sample preparation. Undoubtedly, the most common used are cationic surfactants, among which the most used is the CTAB (51–53) - d: 8.6 nm (52) and 9 nm (53); but some papers have reported the use of anionic surfactants, with SDS the most used (54) - d: 50 nm. Some ILs surfactants such as 1-hexadecyl-3-methylimidazolium bromide have also been used for this purpose (55) - d: 9.1 nm, Ms: 70.53 emu/g. Finally, it should be noted that in some cases these surfactants can be adsorbed onto the surface of a material that is joined to the m-NPs, for example, silica (25), graphene (56) - d: 11 nm, Ms: 53.0 emu/g; or CNTs (41).
Polymer-Coated m-NPs: Polymers have been widely used as sorbents in SPE because many of them are significantly more stable than silica in a larger pH range, giving rise to a greater retention on their structures. Taking advantage of this feature and the fact that some polymers with certain functional groups (carboxylic acids, phosphates, and sulphates) can bind to the surface of iron oxide, in the last few years a large number of works have combined polymers with m-NPs to obtain new sorbents with the high extraction capacity of polymers and the easy separation property of m-NPs (2,16). The binding of polymers to m-NPs can take place by covalent bonding or by electrostatic interactions, creating repulsive forces as a result of the steric hindrance that balance the attractive magnetic, electrostatic, and van der Waals forces acting on the m-NP (2,16).
In the literature it is possible to find research that has used both natural and synthetic polymers. To the best of our knowledge, the most used natural polymer to coat m-NPs is chitosan (57,58) - d: 8–40 nm (57), Ms: 36 emu/g (57); though others, like agarose (59) - d: 90–130 µm; or alginate (60) - Ms: 49.31 emu/g have
also been used. Regarding the use of synthetic polymers, there is more diversity with the most common polyaniline (61) - d: 60 nm; polypyrrole (62); and polydopamine (63) - d: 40 nm. It should be noted that some works which have applied m-NPs coated with more than a single polymer have also been reported in the literature (64,65).
Molecularly Imprinted Polymer-Coated m-NPs: In recent years, molecularly imprinted polymers (MIPs) have proved to be very useful in sample preparation as a result of their high selectivity, especially when complex matrices are analyzed. However, they may suffer some drawbacks (heterogeneous distribution of the binding sites, low binding capacity and selectivity, poor site accessibility, and slow binding kinetics) that can be overcome if they cover the surface of m-NPs (12). Therefore, a new sorbent with both good superparamagnetism and highly selective adsorption capacity is obtained (16). The high selectivity of these materials is provided by threeâdimensional cavities in the polymer’s structure, which are generated by the addition of a template molecule during the synthesis process. For this reason, a wide variety of templates have been used in the literature depending on the structure of the target analytes in each case.
Metal-Organic Framework-Coated m-NPs: MOFs are a class of hybrid organic-inorganic microporous material that can be self-assembled by metal ions with organic linkers through coordination bonds. MOFs have recently been gaining in importance in sample preparation because of their unusual features such as a large surface area, tailorable polarity and pore size, good thermal stability, availability of in-pore functionality, and outerâsurface modification (34,66). These last properties allow MOFs to be combined with m-NPs to create new sorbents with an extraordinary adsoption capacity.
Applications
The wide variety of organic and inorganic materials used as m-NP coatings have increased the range of applicability of these materials in sample preparation and, in particular, in SPE. In this sense, they have been extensively used for the extraction of a widespread number of analytes such as toxic heavy metal ions (19), polycyclic aromatic hydrocarbons (PAHs) (24), and endocrine-disrupting compounds (67) or pesticides (68) among others. The presence of most of these compounds has been particularly studied in samples of low complexity such as different types of water samples (30,31,69).
Among the coatings previously indicated, silica constitutes the one most extensively used because of its versatility to be functionalized. As discussed above, a common modification involves the binding of groups that can act as coordination ligands. Consequently, one of the main applications of silica coated m-NPs has been the extraction of toxic heavy metal ions, particularly from environmental and drinking waters (20,70–72), though samples of biological (19,73) or food origin (19,74) have also been analyzed. However, another important modification that offers a wider field of application involves the binding of C18 to silica. This type of functionalized m-NP has been used, for example, in the extraction of compounds such as phthalates (75), PAHs (76), or pesticides (68), principally from water (77,78) or biological samples (79,80).
Polymer-coated m-NPs have been used to extract organic compounds that play an important negative role in environmental contamination and could produce disorders in humans. In this sense, some of the most relevant analyzed compounds have been pesticide residues (62), PAHs (81), parabens (61), or pharmaceutical compounds (64,82,83) extracted from aqueous (62,81) and biological (64,82) samples in most cases.
As a result of the enhanced features, carbon-coated m-NPs have acquired an important role in analytical chemistry, increasing their applications in recent years. To the best of our knowledge, activated carbon-coated m-NPs have been mainly used for the analysis of organic pollutants (31,84), while CNTs-coated m-NPs (both singleâwalled [SW] CNTs and MWCNTs) have been applied for the extraction of a wider variety of analytes (67,85,86). Graphene-coated m-NPs have been used for the extraction of diverse compounds such as sulphonamides (49), carbamates (87), or PAHs (46). However, and despite physico-chemical differences between the studied compounds, these sorbents have been applied in most cases for the analysis of water samples (46,84,85).
The presence of a surfactant shell provides m-NPs with a higher versatility. In this sense, numerous applications of these modified m-NPs have been reported for the extraction of both organic and inorganic compounds. In fact, they have been used for the extraction and preconcentration of heavy metal ions (88) and different organic contaminants, for example, chlorophenols (55), PAHs (89), or pharmaceutical compounds (53), among others. Environmental water (53) and biological samples (52) have been the most analyzed matrices.
MIP coatings have been generally designed for the extraction of a single target analyte, though the analysis of family-related analytes have also been reported in the last years. Most of these applications include the extraction of endocrine-disrupting substances (90,91) and pharmaceutical compounds (83,92). In this last case, and, as expected, the most studied matrices have been samples of biological origin (90,92).
Despite the advantages offered by MOF-coated m-NPs, to the best of our knowledge, a low number of applications have been reported so far, potentially because of their recent introduction in this field (93,94).
Conclusions
Magnetic nanomaterials have grown in prominence (beyond the analysis of their structural, physical, and morphological characteristics) in a variety of scientific fields. In this sense, in addition to their application in bioscience (as a result of their biocompatibility with biological samples), the number of applications in analytical science, and therefore in extraction methods, has increased enormously. Such an increase in attention has been motivated by the simplification of the extraction step achieved with these materials.
The effectiveness, efficiency, and technological and economic viability of the synthetic procedure have contributed to the extensive use of m-NPs. However, an exhaustive control is required to obtain particles with the desired properties. There are three major challenges for the future growth and use of these type of sorbents: stricter conditions for obtaining them; the instability over long periods of time; and the development of high scale synthesis to make them affordable to commercialize.
The number of applications of m-NPs in extraction methods is expected to grow because, as has been discussed previously, the modification of these particles with different materials not only increases their functionality but also protects the magnetic core.
Finally, it is worth mentioning that the number of studies dealing with the environmental or health impact of the mentioned material is low. Therefore, more studies about the potential effects on health are needed before they can be adopted as routine sorbents. Moreover, the increasing number of applications produces an important amount of residues and further research should be made to ensure the “green” disposal of waste.
References
- L. Gao, J. Wu, S. Lyle. K. Zehr, L. Cao, and D. Gao, J. Phys. Chem. C112, 17357–17361 (2008).
- J. He, M. Huang, D. Wang, Z. Zhang, and G. Li, J. Pharmaceut. Biomed.101, 84–101 (2014).
- G. Giakisikli and A.N. Anthemidis, Anal. Chim. Acta789, 1–16 (2013).
- A.H. Lu, E.L. Salabas, and F. Schüth, Angew. Chem. Ed.46, 1222–1244 (2007).
- J.L. Lyon, D.A. Fleming, M.B. Stone, P. Schiffer, and M.E. Williams, Nano Lett.4, 719–723 (2004).
- J.P. Jolivet and E. Tronc, J. Colloid Interface Sci.125, 688–701 (1988).
- J.L. Benedé, A. Chisvert, D.L. Giokas, and A. Salvador, J. Chromatogr. A1362, 25–33 (2014).
- E.M. Reyes-Gallardo, G. LasarteâAragonés, R. Lucena, S. Cárdenas, and M. Valcárcel, J. Chromatogr. A1271, 50–55 (2014).
- I.P. Román, A. Chisvert, and A. Canals, J. Chromatogr. A1218, 2467–2575 (2011).
- N.M. Mahmoodi, J. Taiwan Inst. Chem. Eng.44, 322–330 (2013).
- S. Kumar, R.R. Nair, P.B. Pillai, S.N. Gupta, M.A.R. Iyengar, and A.K. Sood, ACS Appl. Mater. Interfaces6, 17426–17436 (2014).
- M. Faraji, Y. Yamini, and M. Rezaee, J. Iran. Chem. Soc.7, 1–37 (2010).
- R. Kaur, A. Hasan, N. Iqbal, S. Alam, M.K. Saini, and S.K. Raza, J. Sep. Sci.37, 1805–1825 (2014).
- C. Li, Y. Wei, A. Liivat, Y. Zhu, and J. Zhu, Mat. Lett.107, 23–26 (2013).
- V. Sreeja and P.A. Joy, Mater. Res. Bull.42, 1570–1576 (2007).
- L. Xie, R. Jiang, F. Zhu, H. Liu, and G. Ouyang, Anal. Bioanal. Chem.406, 377–399 (2014).
- C. Huang and B. Hu, J. Sep. Sci.31, 760–767 (2008).
- Y. Deng, D. Qi, C. Deng, X. Zhang, and D. Zhao, J. Am. Chem. Soc.130, 28–29 (2008).
- H. Bagheri, A. Afkhami, M. SaberâTehrani, and H. Khoshsafar, Talanta97, 87–95 (2012).
- M.R. Shishehbore, A. Afkhami, and H. Bagheri, Chem. Cent. J.5, 41–51 (2011).
- N. Zhang, H.Y. Peng, S. Wang, and B. Hu, Microchim. Acta175, 121–128 (2011).
- F. Bianchi, V. Chiesi, F. Casoli, P. Luches, L. Nasi, M. Careri, and A. Mangia, J. Chromatogr. A1231, 8–15 (2012).
- Y. Ji, X. Liu, M. Guan, C. Zhao, H. Huang, H. Zhang, and C. Wang, J. Sep. Sci.32, 2139–2145 (2009).
- S.H. Huo and X.P. Yan, Analyst137, 3445–3451 (2012).
- H. Hea, D. Yuan, Z. Gao, D. Xiao, H. He, H. Dai, J. Peng, and N. Li, J. Chromatogr. A 1324, 78–85 (2014).
- M. Bouri, M. Gurau, R. Salghi, I. Cretescu, M. Zougagh, and Á. Rios, Anal. Bioanal. Chem.404, 1529–1538 (2012).
- X.L. Zhang, H.Y. Niu, W.H. Li, Y.L. Shi, and Y.Q. Cai, Chem. Commun. 47, 4454–4456 (2011).
- X. Zhang, H. Niu, Y. Pan, Y. Shi, and Y. Cai, J. Colloid. Interface Sci.362, 107–112 (2011).
- H. Niu, S. Zhang, X. Zhang, and Y. Cai, ACS Appl. Mater. Interfaces2, 1157–1163 (2010).
- S. Zhang, H. Niu, Z. Hu, Y. Cai, and Y. Shi, J. Chromatogr. A1217, 4757–4764 (2010).
- H. Heidari and H. Razmi, Talanta99, 13–21 (2012).
- Z. Jia, K. Peng, Y. Li, and R. Zhu, Mater. Sci. Eng. B176, 861–865 (2011).
- J. Meng, C. Shi, B. Wei, W. Yu, C. Deng, and X. Zhang, J. Chromatogr. A1218, 2841–2847 (2011).
- J. Tian, J. Xu, F. Zhu, T. Lu, C. Su, and G. Ouyang, J. Chromatogr. A 1300, 2–16 (2013).
- L.M. Ravelo-Pérez, A.V. Herrera-Herrera, J. HernándezâBorges, and M.Á. Rodríguez-Delgado, J. Chromatogr. A1217, 2618–2641 (2010).
- Z. Du, M. Liu, and G. Li, J. Sep. Sci.36, 3387–3394 (2013).
- L. Gao and L. Chen, Microchim. Acta180, 423–430 (2013).
- N. Rastkaria and R. Ahmadkhaniha, J. Chromatogr. A1286, 22–28 (2013).
- Y. Wang, J. Xie, Y. Wu, X. Hu, C. Yang, and Q. Xu, Talanta112, 123–128 (2013).
- S. Xu, C. Jiang, Y. Lin, and L. Jia, Microchim. Acta179, 257–264 (2012).
- D. Xiao, D. Yuan, H. He, C. Pham-Huy, H. Dai, C. Wang, and C. Zhang, Carbon72, 274–286 (2014).
- Q. Ye, L. Liu, Z. Chen, and L. Hong, J. Chromatogr. A1329, 24–29 (2014).
- B. Sha-Sha, L. Zhi, Z. Xiao-Huan, W. Chun, and W. Zhi, Chin. J. Anal. Chem.41, 1177–1182 (2013).
- M. Sun, X. Ma, J. Wang, W. Wang, Q. Wu, C. Wang, and Z. Wang, J. Sep. Sci.36, 1478–1485 (2013).
- S. Zeng, N. Gan, R. Weideman-Mera, Y. Cao, T. Li, and W. Sang, Chem. Eng. J.218, 108–115 (2013).
- Q. Han, Z. Wang, J. Xia, S. Chen, X. Zhang, and M. Ding, Talanta101, 388–395 (2012).
- R.-P. Liang, C.-M. Liu, X.-Y. Meng, J.-W. Wang, and J.-D. Qiu, J. Chromatogr. A1266, 95–102 (2012).
- (X. Cao, L. Shen, X. Ye, F. Zhang, J. Chen, and W. Mo, Analyst 139, 1938–1944 (2014).
- Y.-B. Luo, Z.-G. Shi, Q. Gao, and Y.-Q. Feng, J. Chromatogr. A1218, 1353–1358 (2011).
- M. Moradi and Y. Yamini, J. Sep. Sci.35, 2319–2340 (2012).
- A.A. Rajabi, Y. Yamini, M, Faraji, and S. Seidi, Med. Chem. Res.22, 1570–1577 (2013).
- A. Beiraghi, K. Pourghazi, M. Amoli-Diva, and A. Razmara, J. Chromatogr. B945–946, 46–52 (2014).
- A. Beiraghi, K. Pourghazi, and M. Amoli-Diva, Chem. Eng. Sci.108, 103–110 (2014).
- H. Bagheri, O. Zandi, and A. Aghakhani, Anal. Chim. Acta716, 61–65 (2012).
- Q. Cheng, F. Qu, N.B. Li, and H.Q. Luo, Anal. Chim. Acta715, 113–119 (2012).
- Q. Liu, J. Shi, T. Wang, F. Guo, L. Liu, and G. Jiang, J. Chromatogr. A1257, 1–8 (2012).
- C. Yuwei and W. Jianlong, Chem. Eng. J.168, 286–292 (2011).
- Z. Xu, J. Zhang, L. Cong, L. Meng, J. Song, J. Zhou, and X. Qiao, J. Sep. Sci.34, 46–52 (2011).
- M. Safdarian, P. Hashemi, and M. Adeli, Anal. Chim. Acta774, 44–50 (2013).
- S. Zhang, H. Niu, Y. Cai, and Y. Shi, Anal. Chim. Acta665, 167–175 (2010).
- E. Tahmasebi, Y. Yamini, A. Mehdinia, and F. Rouhi, J. Sep. Sci.35, 2256–2265 (2012).
- Q. Zhao, Q. Lu, and Y.-Q. Feng, Anal. Bioanal. Chem.405, 4765–4776 (2013).
- C. McCullum, P. Tchounwou, L.-S. Ding, X. Liao, and Y.-M. Liu, J. Agric. Food Chem.62, 4261–4267 (2014).
- H. Bagheri, A. Roostaie, and M.Y. Baktash, Anal. Chim. Acta816, 1–7 (2014).
- H. Bagheri, R. Daliri, and A. Roostaie, Anal. Chim. Acta794, 38–46 (2013).
- Y. Wen, L. Chen, J. Li, D. Liu, and L. Chen, TrAC-Trends Anal. Chem. 59, 26–41 (2014).
- J. Ding, Q. Gao, X.S. Li, W. Huang, Z.G. Shi, and Y.Q. Feng, J. Sep. Sci. 34, 2498–2504 (2011).
- Z. Xiong, L. Zhang, R. Zhang, Y. Zhang, J. Chen, and W. Zhang, J. Sep. Sci.35, 2430–2437 (2012).
- H. Heidari, H. Razmi, and A. Jouyban, J. Chromatogr. A1245, 1–7 (2012).
- A. Afkhami, T. Madrakian, R. Ahmadi, H. Bagheri, and M. Tabatabaee, Microchim. Acta175, 69–77 (2011).
- Y. Wang, T. Tian, L. Wang, and X. Hu, Microchim. Acta180, 235–242 (2013).
- H.-M. Jiang, Z.-P. Yan, Y. Zhao, X. Hu, and H.-Z. Lian, Talanta94, 251–256 (2012).
- M.H. Mashhadizadeh and M. Amoli-Diva, J. Anal. At. Spectrom.28, 251–258 (2013).
- A. Carpio, F. Mercader-Trejo, L. Arce, and M. Valcárcel, Electrophoresis33, 2446–2453 (2012).
- Z.B. Li, D. Huang, C.F. Fu, B.W. Wei, W.J. Yu, C.H. Deng, and X.M. Zhang, J. Chromatogr. A 1218, 6232–6239 (2011).
- Y. Liu, H.F. Li, and J.M. Lin, Talanta 77, 1037–1042 (2009).
- B. Maddah and J. Shamsi, J. Chromatogr. A1256, 40–45 (2012).
- C. Jiang, Y. Sun, X. Yu, L. Zhang, X. Sun, Y. Gao, H. Zhang, and D. Song, Talanta 89, 38–46 (2012).
- B. Chu, D.J. Lou, P.F. Yu, S.N. Hu, and S. Shen, J. Chromatogr. A1218, 7248–7253 (2011).
- Q. Wang, L. Huang, P. Yu, J. Wang, and S. Shen, J. Chromatogr. B912, 33–37 (2013).
- E.M. Reyes-Gallardo, R. Lucena, S. Cárdenas, and M. Valcárcel, J. Chromatogr. A 1345, 43–49 (2014).
- A.A. Asgharinezhad, H. Ebrahimzadeh, F. Mirbabaei, N. Mollazadeh, and N. Shekari, Anal. Chim. Acta844, 80–89 (2014).
- H.-B. Zheng, J.-Z. Mo, Y. Zhang, Q. Gao, J. Ding, Q.-W. Yu, and Y.-Q. Feng, J. Chromatogr. A1329, 17–23 (2014).
- H. Niu, Y. Wang, X. Zhang, Z. Meng, and Y. Cai, ACS Appl. Mater. Interfaces4, 286–295 (2012).
- Y.N. Jiao, S.L.Fu, L. Ding, Q. Gong, S.H. Zhu, L.B. Wang, and H. Li, Anal. Methods4, 2729–2734 (2012).
- B. Chen, S. Wang, Q.M. Zhang, and Y.M. Huang, Analyst137, 1232–1240 (2012).
- Q.H. Wu, G.Y. Zhao, C. Feng, C. Wang, and Z. Wang, J. Chromatogr. A1218, 7936–7942 (2011).
- A.E. Karatapanis, Y. Fiamegos, and C.D. Stalikas, Talanta84, 834–839 (2011).
- A. Ballesteros-Gómez and S. Rubio, Anal. Chem.81, 9012–9020 (2009).
- H. Lan, N. Gan, D. Pan, F. Hu, T. Li, N. Long, and L. Qiao, J. Chromatogr. A 1331, 10–18 (2014).
- S. Wang, R. Wang, X. Wu, Y. Wang, C. Xue, J. Wu, J. Hong, J. Liu, and X. Zhou, J. Chromatogr. B905, 105–112 (2012).
- S. Azodi-Deilami, A.H. Najafabadi, E. Asadi, M. Abdouss, and D. Kordestani, Microchim. Acta181, 1823–1832 (2014).
- X. Chen, N. Ding, H. Zang, H. Yeung, R.-S. Zhao, C. Chenga, J. Liua, and T.-W.D. Chan, J. Chromatogr. A1304, 241–245 (2013).
- Y. Wang, J. Xie, Y. Wu, and X. Hu, Microchim. Acta181, 949–956 (2014).
Javier González-Sálamo obtained his BS degree in chemistry at the Universidad de La Laguna (ULL) in Tenerife (Canary Islands, Spain) in 2013 and completed his Master’s degree in chemistry at the same university in 2014. He is currently working to obtain his Ph.D. in chemistry. His research is focused on the development of new methods to extract endocrine-disrupting compounds in environmental, bioanalytical, and food samples making use of several nanomaterials as sorbents in solidâphase extraction and employing chromatographic techniques coupled to different detection systems for their determination. He has recently been awarded a fellowship from the Gobierno Autónomo de Canarias.
Antonio V. Herrera-Herrera obtained his BS degree in chemistry at the Universidad de La Laguna (ULL) in Tenerife (Canary Islands, Spain) in 2007 and received his Ph.D. in chemistry in the same university in 2013, having performed a predoctoral stay at the Institute of Food Science Research (CIAL), which belongs to the Spanish Council for Scientific Research (CSIC) and to Universidad Autónoma de Madrid (UAM). He has recently worked for the General Research Support Service (SEGAI) of the ULL. His research has been primarily focused on the development of new methods to extract and preconcentrate organic contaminants in environmental and food samples by chromatographic and electromigration techniques. During his period as technician at SEGAI, he worked with infrared spectroscopy to analyze samples of different nature. He now works in the Archaeological Micromorphology and Biomarkers Group (AMBI Lab) searching archaeological molecular biomarkers to help to understand human history. He also collaborates with the Department of Chemistry.
Chiara Fanali is associate professor of analytical chemistry at the Department of Medicine, Università Campus BioâMedico of Rome (Italy). In 2008 she received her Ph.D. degree in biochemical studies of proteomics at Catholic University of Rome (Italy). Since February 2010 she has performed her research at Università Campus Bio-Medico in Rome. Her main research interests focus on the application of modern and innovative analytical techniques to analyze and characterize food bioactive compounds and peptides, as well as proteins in biological fluids. Techniques include high performance liquid chromatography (HPLC) and nano-liquid chromatography coupled to mass spectrometers, including ion trap (IT), single quadrupole, and high resolution linear ion trapâOrbitrap time-of-flight (TOF) mass spectrometry.
Javier Hernández-Borges is associate professor of analytical chemistry at the Department of Chemistry of the Universidad de La Laguna (ULL) in Tenerife (Canary Islands, Spain). In 2005 he received his PhD degree in chemistry at that university, performing several preâdoctoral stays and a post-doc stay at the Institute of Chemical Methodologies of the Italian National Research Council (CNR) in Monterotondo (Rome, Italy). His research is focused at this time on the development of new analytical methodologies for the extraction and preconcentration of pesticides, estrogenic compounds, and plastic migrants in environmental, bionalytical, and food samples by chromatographic and electromigration techniques, also using m-NPs.


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








