Practical Advances in SFC for the Purification of Pharmaceutical Molecules
Recent progress in supercritical fluid chromatography (SFC) instrumentation has led to renewed interest in the technique as a powerful tool for chiral and achiral separations of pharmaceutical molecules, for both analytical and preparative purposes. The “green” aspect of the technique and low running costs make SFC technology particularly attractive for preparative chromatography because it considerably reduces the consumption of organic solvents. These factors led to a revised strategy for purification and to a general interest in evaluating possible extensions for the application of packed SFC (pSFC). The results of this extensive evaluation have led to the establishment of SFC platforms for preparative achiral purifications as a standard practice, alongside its use for preparative chiral separations.
Photo Credit: Jorg Greuel/Getty Images

Eric Francotte, Novartis Institutes for Biomedical Research, Discovery Chemistry, Basel, Switzerland.

Recent progress in supercritical fluid chromatography (SFC) instrumentation has led to renewed interest in the technique as a powerful tool for chiral and achiral separations of pharmaceutical molecules, for both analytical and preparative purposes. The “green” aspect of the technique and low running costs make SFC technology particularly attractive for preparative chromatography because it considerably reduces the consumption of organic solvents. These factors led to a revised strategy for purification and to a general interest in evaluating possible extensions for the application of packed SFC (pSFC). The results of this extensive evaluation have led to the establishment of SFC platforms for preparative achiral purifications as a standard practice, alongside its use for preparative chiral separations.
Supercritical fluid chromatography (SFC) has been around for a long time (1) but the real interest in packed SFC (pSFC) or SFC using packed columns began in the early 1980s when the first commercial preparative instruments became available (2). The parallel development of efficient chiral stationary phases and the availability of more robust preparative SFC instruments led to a growing interest in pSFC to separate stereoisomers, which started with the pioneering work of Marcel Caude and his group in 1985 (3). Chiral separations rescued SFC technology from becoming obsolete.
SFC can also be regarded as a “green” technique. It is also worth highlighting in relation to the “greenness” of the technique that SFC does not produce CO2, it uses existing CO2, thus indirectly reducing the environmental impact of organic solvent waste that is usually burnt after use in other chromatographic techniques.
pSFC has now been adopted for preparative purposes by most pharmaceutical companies to perform chiral separations from the mg- to kg-scale. This growth ran in tandem with regular improvements in SFC instrumentation.
SFC and Chiral Separations
pSFC is now commonly used for preparative chiral separations in the drug discovery environment because it can rapidly process a lot of samples in relatively small amounts. For very large amounts - tens of kilograms or tons - other techniques such as continuous multiâcolumn chromatography (SMB, varicol) are still preferred (4–7). Preparative pSFC has been fully adopted in many research laboratories (8–14), because SFC has numerous advantages, including high diffusivity, low pressure drops, short equilibration times, reduced solvent consumption, and, in preparative applications, fast solvent removal. SFC also offers lower solvent costs, less safety concerns with respect to flammability and toxicity, and reduced impact on the environment. These advantages apply to both preparative chiral separation and achiral purification.
The general chiral SFC process flow in our laboratories is the same as for chiral liquid chromatography (HPLC) separations, which involves chiral method development, performance of the preparative separation, and purity control of the collected fractions.
For chiral method development, several groups have reported on their own process flow (15–17) but the chiral column market is continuously evolving, so the setup has to be regularly adapted, even though the preferred columns remain those based on polysaccharides. Most users have adopted an automated serial column screening system or a parallel device, which can test up to five columns at once. For over five years, our standard setting has consisted of a highâperformance parallel chiral screening SFC instrument that is capable of operating eight columns in parallel simultaneously. This setup is similar to what we implemented earlier for chiral HPLC (18) and rapidly identifies optimal conditions (chiral column, mobile phase, flow rate) to perform the preparative separation for mg to several hundreds of grams amounts. As an example, Figure 1 shows the screening results of the chiral screening of a racemic chiral imidazole derivative on eight different chiral columns with six different mobile phases (48 different conditions tested within 42 min). This illustrates the effectiveness of this parallel approach.
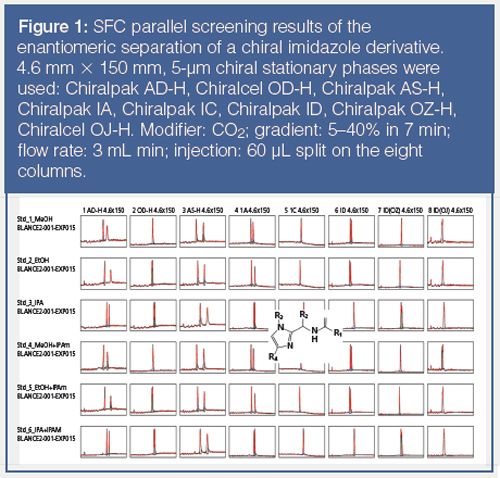
The most used chiral stationary phases in pSFC (and also in chiral HPLC) are those based on the polysaccharides cellulose and amylose. Our standard screening set of columns includes a number of immobilized polysaccharide derivatives that we developed over the last decade (18–22).
For preparative chiral pSFC separations, instruments with the capacity to operate at flow rates of 10 mL/min to several hundreds of mL/min are available to meet the requirements needed to work at various scales. As a result of the low viscosity of supercritical CO2, small particle size columns can be operated, which means high efficiencies can be obtained while maintaining high flow rates.
Usually the stacked injection technique is applied. This technique permits the use of relatively smaller columns compared with HPLC, while optimizing the productivity (Figure 2). Figure 2 shows the chromatogram of the preparative pSFC resolution of 50 g of racemic 1-benzyl-3-methyl-2-piperidone, a drug intermediate, on a chiral column. Ethanol (5%) was used as the modifier in the mobile phase and the separation was performed under isocratic condition. The enantiomeric excess of the separated enantiomer was greater than 99%. This application demonstrates the high efficiency of pSFC, which can reach very respectable productivity, up to 2.3 kg racemate/kgCSP/day in this case.
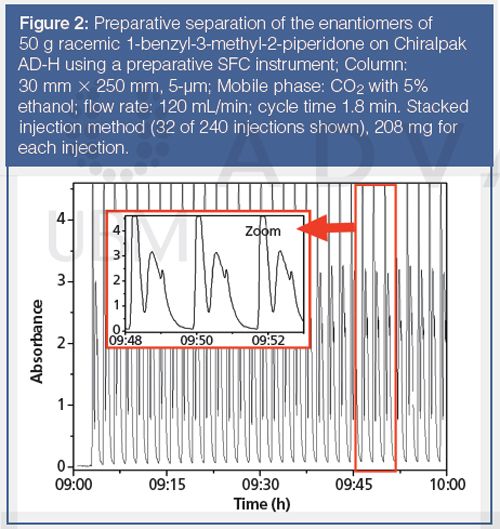
With the new generation of chiral columns based on immobilized polysaccharides, it is possible to apply strong solvating modifiers like ethyl acetate, tetrahydrofuran, dichloromethane, chloroform, or dimethoxymethane (DMME), which are not tolerated by the classical non-immobilized polysaccharide phases (21–22). This is a great advantage and (like chiral HPLC) it permits the retention times and selectivity to be modulated, and the solubility of the sample to be improved, which is often a major issue in preparative separation/purification of drug and drug intermediates. Figure 3 shows the chiral SFC separation of a few drugs using immobilized polysaccharide phases and CO2 mixed with “non-classical” modifiers.
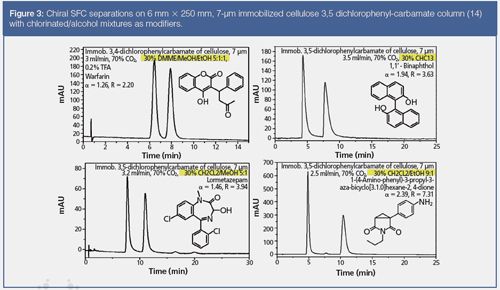
The use of chiral stationary phases under SFC mode is not restricted to the separation of enantiomers, but it is also regularly applied in our laboratories for the separation of diastereoisomers, metabolites, or simply regioisomers. An example of application to the separation of regioisomers is shown in Figure 4 for two iodophenyl compounds.
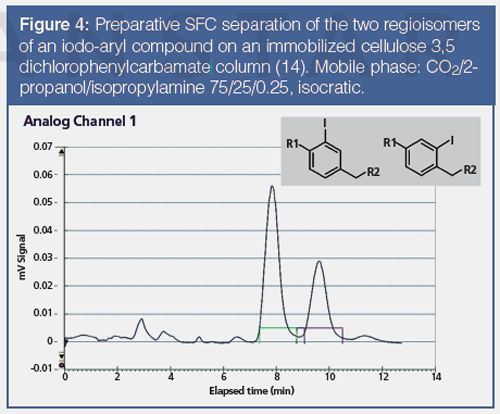
In terms of solvent consumption and costs, chiral SFC clearly shows considerable advantages compared to chiral HPLC. It has been estimated that on average the consumption of organic solvents is reduced by 60–70% and the running costs by 70–80%. Even though pSFC is the method of choice and is able to resolve almost all racemic compounds on an analytical scale, chiral HPLC remains a complementary technique because there are some compounds that are not separated by SFC or much better by HPLC, or which are barely soluble under SFC conditions.
pSFC Versus Reversed-Phase HPLC for Achiral Purifications
A limited number of applications of achiral preparative purifications have been published between 1980 and 2000, but these were very sporadic (23,24). In this time frame, reversed-phase HPLC made significant progress and developed into a very efficient and generally applicable technique for achiral purification, although normal phase chromatography is still intensively used in many research laboratories in the flash chromatography mode. Reversedâphase HPLC had the advantage of predominantly using a quasi-universal stationary phase (C18) and a generally applicable mobile phase mixture (acetonitrile/water). Reversed-phase HPLC is also very compatible with mass spectrometry (MS) detection. These reasons have made reversed-phase HPLC–MS the standard approach for the purification of small amounts (a few mg to grams) in pharmaceutical research and in many other research environments.
A few research groups have successfully developed their own pSFC–MS purification system, in particular in the context of combinatorial chemistry projects (25–27), but these instruments were not accessible to the global community of separation scientists and were limited in their applicability with respect to sample size. For years, it has been a huge challenge to develop a robust and reliable preparative
pSFC–MS instrument that also offers mass-directed fractionation. This has been a considerable curb to the development of preparative pSFC for achiral purification of complex mixtures for which mass-directed fractionation is a must.
A dedicated preparative pSFC–MS instrument was introduced in 2009, and using a prototype, we evaluated the possibility of extending pSFC technology to achiral purifications, in particular as a replacement for the very popular reversed-phase chromatography method that uses acetonitrile. The details of this preliminary evaluation were reported elsewhere (28). Sample mixtures (100 mg) of small synthetic libraries and from single reactions were injected on a preparative reversed-phase HPLC–MS and a SFC–MS instrument. The performance of both techniques was assessed and compared with respect to purity, speed, and recovery.
It was found that, on average, pSFC is comparable to reversed phase chromatography in terms of recovery and purity, although recovery was better in pSFC for some compounds and better in reversed-phase HPLC for other compounds. However, on average SFC was three times faster than reversed-phase HPLC. The introduction of the dedicated preparative SFC–MS instrument prompted other groups to perform independently similar evaluation studies (29-31).
Expanding on our results, we built several SFC–MS-directed fractionation platforms within our company to support medicinal chemists in their efforts to develop new drugs (32). The focus is on the purification of drug candidates that are generally obtained from complex synthetic steps and therefore require full attention and no compromise with respect to recovery and purity. This requirement involves the development of a “tailor-made” purification approach for each individual sample.
This is different in the “hit-finding” phase where large libraries are produced and must be purified very quickly using generic methods passing on analytical screening of various columns and accommodating lower recovery and purity.
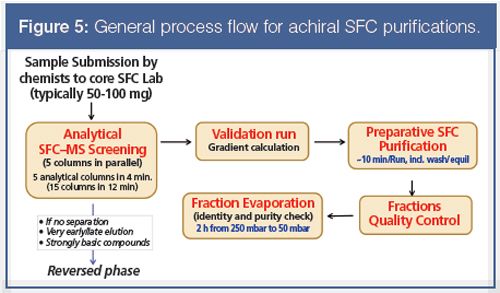
The established general strategy of purification of our compounds is presented in Figure 5. Today, the strategy permits the rapid and efficient purification of several thousands of samples every year in our laboratories, mostly final products to be submitted to biological testing. Currently, about 80% of the submitted compounds are purified by pSFC. This is a radical change.
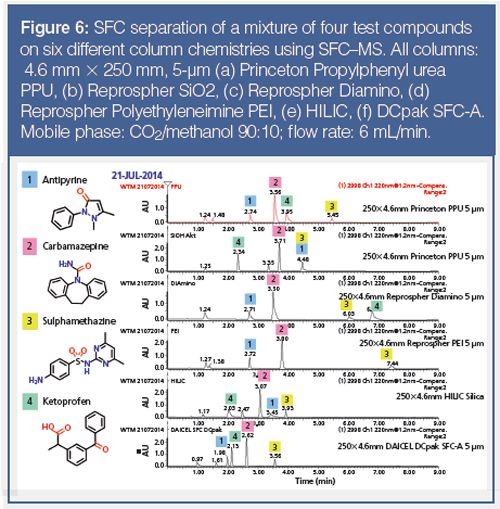
Unlike reversed-phase HPLC, the identification of the right column chemistry is critical for the successful application of achiral pSFC. This is illustrated on Figure 6, which shows the SFC separation of a mixture of four test compounds on six different column chemistries. Figure 6 clearly demonstrates that very different selectivity can be achieved depending on the column chemistry. Basic, neutral, and acid compounds are well eluted on most columns, indicating the suitability of pSFC for a broad range of chemical functionalities. Compound 4 on the basic polyethyleneimine (PEI) phase is an exception, probably because of an excessively strong interaction between the acidic solute and the basic stationary
phase.
The use of a variety of columns might be seen as a disadvantage compared to reversed-phase HPLC, which mostly applies the C18 “universal” column. However, it can also be seen as an opportunity to optimize selectivity for a particular compound. In this respect, the approach is similar to the one applied for chiral separations for which each preparative resolution first needs the screening of a set of analytical columns. Column manufacturers have obviously realized the opportunity of this emerging market and the number of “SFC” columns for achiral purifications has
grown rapidly in the past three years - and continues to grow.
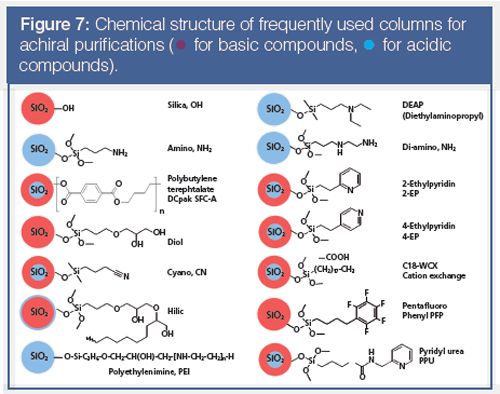
The chemical functionality of the most frequently used columns in our laboratories is shown in Figure 7, but these columns could be quite different in a few years because stationary phase development is currently very dynamic in this area. Nevertheless, a small number of column chemistries could emerge as the preferred columns in the future, which is what happened with chiral phases. Our experience (with over tens of thousands samples purified) shows that the columns can be classified according to their applicability, some being more suitable for basic or acidic compounds, and some accommodating both types of molecules (Figure 7) (33). This is an empirical observation that complements the more theoretical classification approach developed by Caroline West et al. (34).
As the retention of the single components of a mixture on a defined stationary phase cannot be predicted, it is important to rely on an efficient screening platform to maximize the quality of the separation with respect to the target substance. As the molecular mass of the molecule is a critical consideration for selecting the optimal column with respect to the target compound to be purified, the analytical screening must be very effective and also performed by SFC–MS. This can be easily done using an SFC–MS system equipped with five columns in parallel.
When the bank of the preferred five columns is not successful, there is an option to switch to a second or third bank of five columns (Figure 5). After identification of the best column chemistry, a validation run is performed to collect the data needed to calculate the optimal gradient for the preparative purification. This is necessary because there is no controller regulating the flow-rate in the individual columns on the SFC–MS parallel screening unit used.
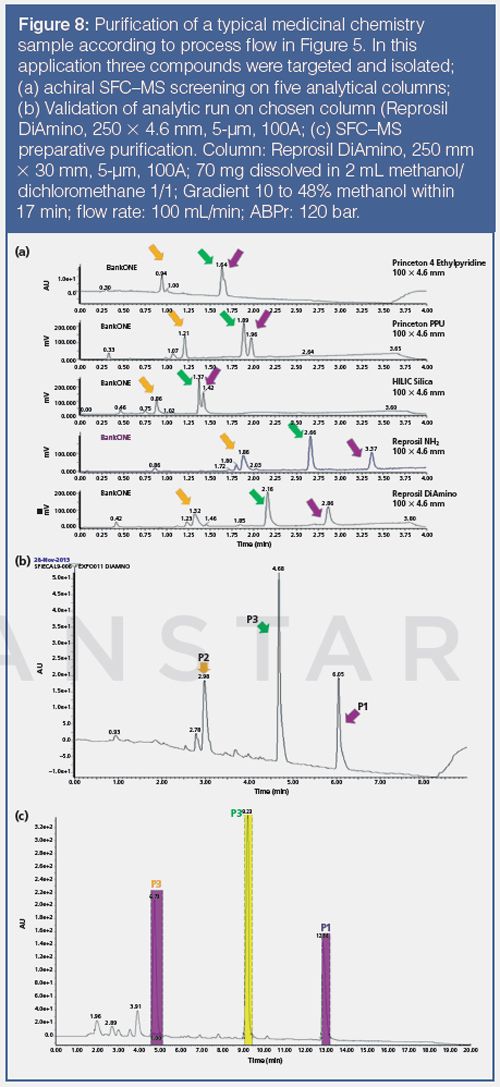
Figure 8 shows a typical application of an achiral pSFC purification, from the analytical column screening to the final preparative separation. In this case two banks of columns (10 columns in total) were screened, but usually one bank is sufficient (Figure 8[a]). After identification of the best column (in this case a diaminopropyl modified silica gel column), a validation test is performed with the same column to determine the optimal gradient conditions for the preparative purification (Figure 8[b]). We developed a simple gradient calculation procedure. The calculation procedure is applicable for all column chemistries, but is specific for a particular type of screening instrument, column size, and flow-rate. Figure 8(c) demonstrates the good extrapolation between the validation analytical run and the preparative run.
At the moment achiral SFC replaces reversed-phase HPLC for at least 75% of the applications related to the purification of small molecules in our laboratory. The application range is continuously expanding and covers drug molecules and intermediates, natural products, metabolites, or small linear and cyclo-peptides.
Further advantages of pSFC over reversed-phase HPLC in preparative applications include much lower energy consumption (7 times less) for solvent removal. In addition, it is desirable, whenever possible, to avoid the use of acetonitrile which is highly toxic because it metabolizes into cyanhydric acid in the body after inhalation or skin penetration through simple contact.
Conclusion
pSFC has now overcome a lot of obstacles that were previously associated with it for preparative chromatography as a result of advances in modern preparative SFC instruments.
The successful implementation of preparative pSFC platforms in our laboratories has changed our achiral purification strategy. SFC is now the technique of first choice with reversed phase chromatography becoming the second option. Reversed-phase HPLC is still used but only for the samples that are not appropriate for processing by SFC (about 20%).
Even though the potential of SFC for achiral purification has not been fully exploited, tens of thousands of samples are purified every year with an average turnaround time of 24 h, including evaporation, drying, and quality control, which is usually performed by reversed-phase HPLC as an orthogonal checking method. The speed of the technique, the high purity of the isolate substances, and the absence of trifluoroacetic acid (TFA), which is known to be toxic, are benefits that are appreciated by the medicinal chemists.
For reversed-phase HPLC, the environmental impact of the switch to pSFC is smaller than for normal phase applications because the amount of organic solvent is only partially reduced, with methanol replacing acetonitrile. Nevertheless, the switch remains “greener” because it consumes on average about 20% less organic solvents and methanol is generally considered to be “greener” than acetonitrile.
We anticipate that pSFC will replace reversed-phase HPLC for other applications (strongly basic compounds, larger molecules, biomolecules). Even though reversed-phase HPLC will remain a complementary purification technique, pSFC is clearly changing the face of the purification world. If it fits the separation purpose, SFC should be the preferred technique considering the reduced environmental impact and cost. In this context, it should be also highly encouraged to explore further possible application fields for SFC.
References
- E. Klesper, A.H. Corwin, and D.A. Turner, J. Org. Chem.27, 700–701 (1962).
- T.A. Berger, K. Fogleman, T. Staats, P. Bente, I. Crocket, W. Farrell, and M. Osonubi, J. Biochem. Biophys. Meth.43, 87–111 (2000).
- P. Mourier, E. Eliot, M. Caude, R. Rosset, A. Tambute, Anal. Chem.57, 2819–2823 (1985).
- M. Juza, M. Mazzotti, and M. Morbidelli, Trends in Biotechnology18, 108–118 (2000).
- L. Miller, C. Orihuela, R. Fronek, D. Honda, and O. Dapremont, J. Chromatogr. A849, 309–317 (1999).
- R.-M. Nicoud and R.E. Majors, LCGC18, 680–687 (2000).
- E. Francotte and P. Richert, J. Chromatogr. A769, 101–107 (1997).
- M. Villeneuve, R. Schmidt, Y. Zhao, “Introduction of unique Analytical and Preparative SFC units, paper presented at the 5th International Conference on Packed-Column SFC”, New York, USA, 20–22 July 2011. http://greenchemistrygroup.org/pdf/2011/Villeneuve_SFC_2011_Presentation.pdf
- J. Van Anda, Am. Pharm. Rev.12, 48–53 (2009).
- L. Miller and M. Potter, J. Chromatogr. B875, 230–236 (2008)
- L. Miller, J. Chromatogr. A1250, 250–255 (2012).
- D-R. Wu, L. Leith, B. Balasubramanian, T. Palcic, and D. WangâIverson, Am. Laborat.38, 24–26 (2006).
- E. Seest, M. Belvo, T. Perun, P. McDermott, “Green Lab Scale Chiral Chromatography: Comparison of Purification Techniques for Cost Effective and Efficient Processing of Samples While Minimizing Environmental Impact, paper presented at the 5th International Conference on Packed-Column SFC”, New York, USA, 20–22 July 2011. http://greenchemistrygroup.org/pdf/2011/Seest_SFC_2011_Presentation.pdf
- C. Welch, W. Leonard, J. DaSilva, M. Biba, J. Albaneze-Walker, D. Henderson, B. Laing, and D. Mathre, LCGC Europe18, 264–272 (2005).
- C.J. Welch, M. Biba, J.R. Gouker, G. Kath, P. Augustine, and P. Hosek, Chirality19, 184–189 (2007).
- D. Mangelings and Y. Vander Heyden, J. Sep. Sci.31, 1252–1273 (2008).
- C. Hamman, M. Wong, I. Aliagas, D.F. Ortwine, J. Pease, D.E. Schmidt, and J. Victorino, J. Chromatogr A.1305, 310–319 (2013).
- E. Francotte, D. Huynh, and H. Wetli, G.I.T. Laboratory Journal Europe10, 46–48 (2006).
- E. Francotte, Patent, PCT Int. Appl. WO 9749733, 1997.
- E. Francotte and T. Zhang, Patent, PCT Int. Appl. WO 9704011, 1997.
- E. Francotte and D. Huynh, J. Pharm. Biomed. Anal. 27, 421–429 (2002).
- E. Francotte and G. Diehl, “Extending the SFC applicability in the field of enantioselective separations”, paper presented at the 2nd International Conference on Packed-Column SFC, Zurich, Switzerland, 1–2 October 2008. http://greenchemistrygroup.org/pdf/2008/DO0900Francotte.pdf
- K. Anton and C. Berger, Eds., Supercritical Fluid Chromatography with Packed Columns (Marcel Dekker, Inc., New York, USA, 1998).
- L.T. Taylor, J. Supercritical Fluids47, 566–573 (2009).
- X. Zhang, M.H. Towle, C.E. Felice, J.H. Flament, and W.K. Goetzinger, J. Comb. Chem.8, 705–714 (2006).
- P.A. Searle, K.A. Glass, and J.E. Hochlowski, J. Comb. Chem.6, 175–80 (2004).
- T. Wang, M. Barber, I. Hardt, and D.B. Kassel, Rapid Com. Mass Spectr.15, 2067–2075 (2001).
- E. Francotte and G. Diehl, “SFC- The Universal Separation Technique?”, paper presented at the 4th International Conference on Packed-Column SFC, Stockholm, Sweden, 15–16 September 2010. http://greenchemistrygroup.org/pdf/2010/Francotte.pdf
- V. Lazarescu, M.J. Mulvihill, and L. Ma, LCGC North America 29, 438–444 (2011).
- J. Van Anda, Am. Pharm. Rev. 13, 111–115 (2010).
- K. Ebinger, H.N. Weller, J. Kiplinger, and P. Lefebvre, J. Lab. Automation16, 241–249 (2011).
- E. Francotte, I. Adam, T. Mann, and T. Wolf, “SFC Evolving as the Prevailing Purification Technique in Medicinal Chemistry”, paper presented at the 6th International Conference on Packed-Column SFC, Brussels, Belgium, 4–5 October 2012. http://greenchemistrygroup.org/pdf/2012/Francotte.pdf
- E. Francotte, “Why Should SFC Be the First Choice Technology for the Purification of Pharmaceuticals?”, paper presented at the 8th International Conference on Packed-Column SFC, Basel, Switzerland, 2014. http://greenchemistrygroup.org/pdf/2014/Francotte_SFC%202014%20Presentation.pd
- C. West, M.A. Khalikova, E. Lesellier, and K. Heberger, J. Chromatogr. A1409, 241–250 (2015).
Dr. Eric Francotte is currently responsible for a technology platform dealing with separation sciences at Novartis Research (Basle, Switzerland). After completion of his PhD in organic chemistry in Belgium and a postdoc at the Geneva University, he joined former Ciba-Geigy (Novartis) where he established a centre of expertise for the development and application of chiral polymers for the chromatographic resolution of chiral compounds. His major achievements include the invention of an innovative process to immobilize polysaccharide derivatives as chiral stationary phases. Several phases arising from this technology have been marketed as the result of an external collaboration and are now used in almost all laboratories dealing with chiral separations in the industry and academia in the world. He was also a pioneer for the implementation of new chromatographic technologies such as simulated-moving-bed and supercritical fluid chromatography in the pharmaceutical industry. He is the author or co-author of numerous publications, patents, and editor of several books. He chaired various International Symposia in the field of preparative chromatography, chirality, and SFC.

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








